NDC Code(s) : 0338-3508-41, 0338-3503-41
Packager : Baxter Healthcare Corporation
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| CefazolinCefazolin Sodium INJECTION, SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| CefazolinCefazolin Sodium INJECTION, SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| LABELER - Baxter Healthcare Corporation(005083209) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Baxter Healthcare Corporation | 194684502 | ANALYSIS(0338-3508, 0338-3503), LABEL(0338-3508, 0338-3503), MANUFACTURE(0338-3508, 0338-3503), PACK(0338-3508, 0338-3503), STERILIZE(0338-3508, 0338-3503) | |
PRINCIPAL DISPLAY PANEL
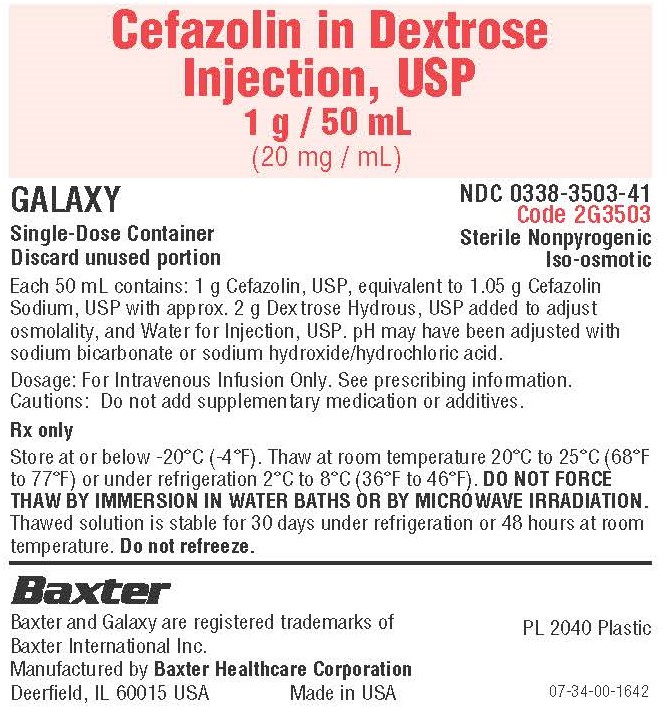
 Container Label
Container Label
Cefazolin in Dextrose
Injection, USP
1 g / 50 mL
(20 mg / mL)
GALAXY
Single Dose Container
Discard unused portion
NDC 0338-3503-41
Code 2G3503
Sterile Nonpyrogenic
Iso-osmotic
Each 50 mL contains: 1 g Cefazolin, USP, equivalent to 1.05 g Cefazolin
Sodium, USP with approx. 2 g Dextrose Hydrous, USP added to adjust
osmolality, and Water for Injection, USP. pH may have been adjusted with
sodium bicarbonate or sodium hydroxide/hydrochloric acid.
Dosage: For Intravenous Infusion Only. See prescribing information.
Cautions: Do not add supplementary medication or additives.
Rx only
Store at or below -20°C (-4°F). Thaw at room temperature 20°C to 25°C (68°F to
77°F) or under refrigeration 2°C to 8°C (36°F to 46°F). DO NOT FORCE
THAW BY IMMERSION IN WATER BATHS OR BY MICROWAVE IRRADIATION.
Thawed solution is stable for 30 days under refrigeration or 48 hours at room
temperature. Do not refreeze.
Baxter Logo
Baxter and Galaxy are registered trademarks of
Baxter International Inc.
Manufactured by Baxter Healthcare Corporation
Deerfield, IL 60015 USA
Made in US
PL2040 Plastic
07-34-00-1642
* BAR CODE POSITION
ONLY
303383503411

 Carton Label
Carton Label
Thaw at room temperature 20°C to 25°C (68°F to 77°F) or under refrigeration 2°C to 8°C (36°F to 41°F).
DO NOT FORCE THAW BY IMMERSION IN WATER BATHS OR BY MICROWAVE IRRADIATION. Thawed solution is stable for 30 days under refrigeration or 48 hours at room temperature.
Do not refreeze.
Handle frozen product containers with care. Product containers may be fragile in the
frozen state.
Baxter and Galaxy are registered trademarks of Baxter International Inc.
PL 2040 Plastic
07-04-00-0801
Cefazolin in Dextrose Injection, USP
1 g / 50 mL (20 mg / mL)
Contains 12 units of Single Dose bags. Each bag contains 50 mL Iso-osmotic
Store at or below -20°C (-4°F). Do not refreeze.
Rx Only
NDC 0338-3503-41
Code 2G3503
*FOR BAR CODE POSITION ONLY
(01) 20303383503415
GALAXY Container
Sterile Nonpyrogenic
Each 50 mL contains: 1g Cefazolin, USP, equivalent to 1.05 g Cefazolin Sodium, USP with approx. 2 g
Dextrose Hydrous, USP added to adjust osmolality, and Water for Injection, USP. pH may have been adjusted
with sodium bicarbonate or sodium hydroxide/hydrochloric acid.
Dosage: For Intravenous Infusion Only. See prescribing information.
Cautions: Do not add supplementary medications or additives.
Baxter Logo
Baxter and Galaxy are registered trademarks of Baxter International, Inc.
Baxter Healthcare Corporation Deerfield, IL 60015 USA

 Container Label
Container Label
Cefazolin in Dextrose
Injection, USP
2 g / 100 mL
(20 mg / mL)
GALAXY
Single Dose Container
Discard unused portion
NDC 0338-3508-41
Code 2G3508
Sterile Nonpyrogenic
Iso-osmotic
Each 100 mL contains: 2 g Cefazolin, USP, equivalent to 2.1 g Cefazolin
Sodium, USP with approx. 4 g Dextrose Hydrous, USP added to adjust
osmolality, and Water for Injection, USP. pH may have been adjusted with
sodium bicarbonate or sodium hydroxide/hydrochloric acid.
Dosage: For Intravenous Infusion Only. See prescribing information.
Cautions: Do not add supplementary medication or additives.
Rx only
Store at or below -20°C (-4°F). Thaw at room temperature 20°C to 25°C (68°F to
77°F) or under refrigeration 2°C to 8°C (36°F to 46°F). DO NOT FORCE THAW
BY IMMERSION IN WATER BATHS OR BY MICROWAVE IRRADIATION. Thawed
solution is stable for 30 days under refrigeration or 48 hours at room
temperature. Do not refreeze.
Baxter Logo
Baxter and Galaxy are registered trademarks of Baxter International Inc.
Manufactured by Baxter Healthcare Corporation Deerfield, IL 60015 USA
Made in USA
PL2040 Plastic
07-34-00-1643
* BAR CODE POSITION
ONLY
303383508416

 Carton Label
Carton Label
Thaw at room temperature 20°C to 25°C (68°F to 77°F) or under refrigeration 2°C to 8°C (36 °F to 41°F).
DO NOT FORCE THAW BY IMMERSION IN WATER BATHS OR BY MICROWAVE IRRADIATION. Thawed solution is stable for 30 days under refrigeration or 48 hours at room temperature.
Do not refreeze.
Handle frozen product containers with care. Product containers may be fragile in the
frozen state.
PL 2040 Plastic
07-04-00-0802
Cefazolin in Dextrose Injection, USP
2 g / 100 mL (20 mg/ mL)
Contains 6 units of Single Dose bags. Each bag contains 100 mL. Iso-osmotic
Store at or below -20°C (4°F). Do not refreeze.
Lot XXXXXX Exp. DD MMM YY
Rx Only
NDC 0338-3508-41
Code 2G3508
*FOR BAR CODE POSITION ONLY
(01) 20303383508410
GALAXY Container
Sterile Nonpyrogenic
Each 100 mL contains: 2 g Cefazolin, USP, equivalent to 2.1 g Cefazolin Sodium, USP with approx. 4 g
Dextrose Hydrous, USP added to adjust osmolality, and Water for Injection, USP. pH may have been
adjusted with sodium bicarbonate or sodium hydroxide/hydrochloric acid.
Dosage: For Intravenous Infusion Only. See prescribing information.
Cautions: Do not add supplementary medications or additives.
Baxter Logo
Baxter and Galaxy are registered trademarks of Baxter International, Inc.
Baxter Healthcare Corporation Deerfield, IL 60015 USA









