NDC Code(s) : 0363-0521-62
Packager : Walgreen Company
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| IbuprofenIbuprofen TABLET, CHEWABLE | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
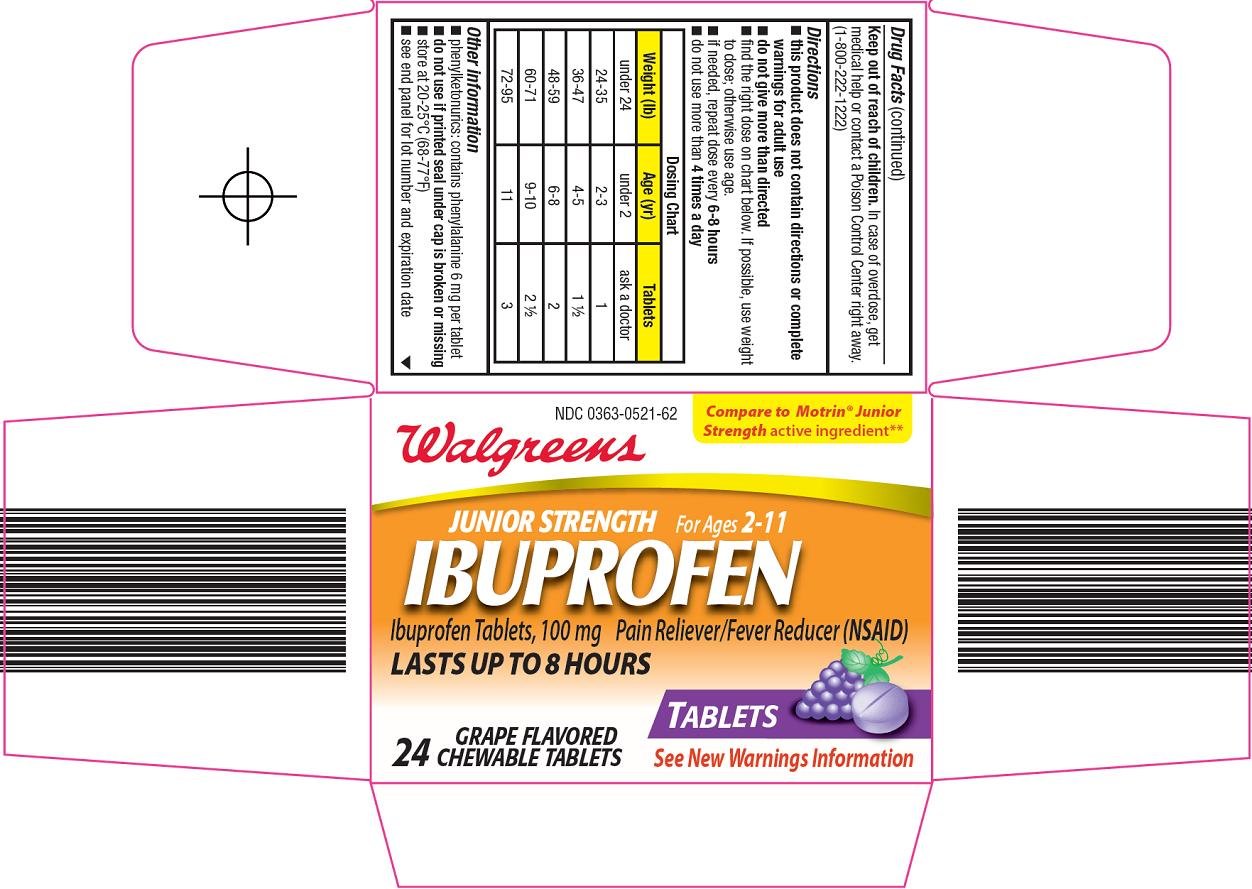
PRINCIPAL DISPLAY PANEL
Compare to Motrin® Junior Strength active ingredient
Junior Strength
For Ages 2-11
IBUPROFEN
Ibuprofen Tablets, 100 mg
Pain Reliever/Fever Reducer (NSAID)
LASTS UP TO 8 HOURS
TABLETS
See New Warnings Information
 Ibuprofen Carton Image 1
Ibuprofen Carton Image 1
 Ibuprofen Carton Image 2
Ibuprofen Carton Image 2









