NDC Code(s) : 0374-5090-01
Packager : Lyne Laboratories, Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Fluocinolone Acetonide Fluocinolone Acetonide Oil OIL | |||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
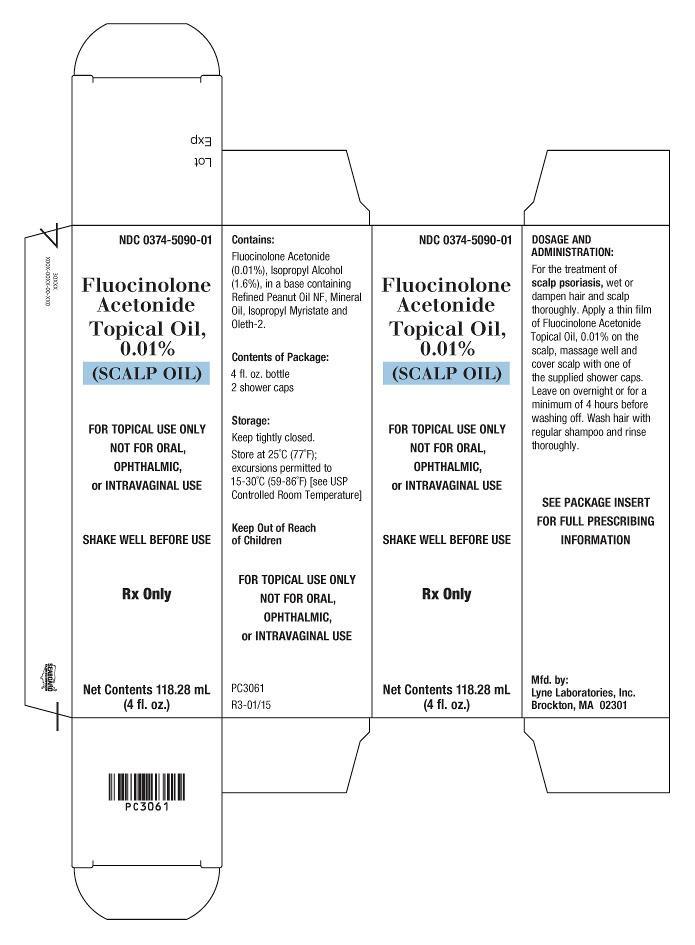
PRINCIPAL DISPLAY PANEL
NDC 0374-5090-01
Fluocinolone
Acetonide
Topical Oil,
0.01%
(SCALP OIL)
FOR TOPICAL USE ONLY
NOT FOR ORAL,
OPHTHALMIC,
or INTRAVAGINAL USE
SHAKE WELL BEFORE USE
Rx Only
Net Contents 118.28 mL
(4 fl. oz.)