NDC Code(s) : 0378-0833-35, 0378-0834-35
Packager : Mylan Pharmaceuticals Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Amoxicillin and Clavulanate PotassiumAmoxicillin and Clavulanate Potassium POWDER, FOR SUSPENSION | ||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Amoxicillin and Clavulanate PotassiumAmoxicillin and Clavulanate Potassium POWDER, FOR SUSPENSION | ||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
PRINCIPAL DISPLAY PANEL
NDC 0378-0833-35
Amoxicillin and
Clavulanate
Potassium for
Oral Suspension, USP
200 mg/28.5 mg
(per 5 mL)
When reconstituted, each 5 mL
contains:
AMOXICILLIN, 200 mg,
as the trihydrate
CLAVULANIC ACID, 28.5 mg,
as clavulanate potassium
Rx only 100 mL (when reconstituted)
Use only if inner seal is intact.
Net contents: Equivalent to 4 g amoxicillin and
0.57 g clavulanic acid. Each 5 mL of reconstituted
amoxicillin and clavulanate potassium 200 mg/
28.5 mg/5 mL oral suspension contains 0.14 mEq
potassium. Store dry powder at 20°-25°C
(68°-77°F) [See USP Controlled Room Temperature].
Directions for mixing: Tap bottle until all powder
flows freely. Add approximately 2/3 of total water for
reconstitution (total = 88 mL); shake vigorously to wet
powder. Add remaining water, again shake vigorously.
Dosage: Administer every 12 hours. See accompanying
prescribing information.
Phenylketonurics: Contains phenylalanine 7 mg per
5 mL. Keep tightly closed. Shake well before using.
Must be refrigerated. Discard after 10 days.
Manufactured for:
Mylan Pharmaceuticals Inc.
Morgantown, WV 26505 U.S.A.
Manufactured in Austria
46178949
RSDZ0833KF1

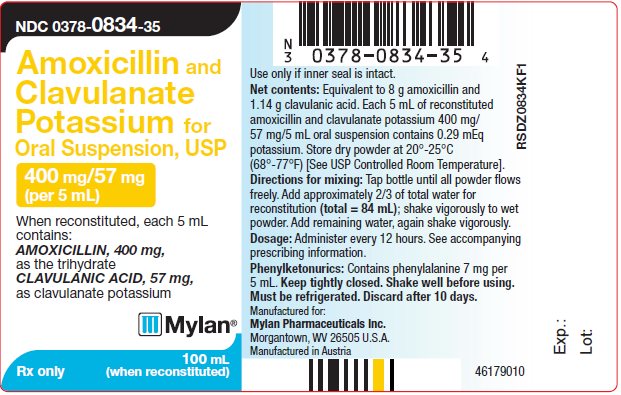
PRINCIPAL DISPLAY PANEL
NDC 0378-0834-35
Amoxicillin and
Clavulanate
Potassium for
Oral Suspension, USP
400 mg/57 mg
(per 5 mL)
When reconstituted, each 5 mL
contains:
AMOXICILLIN, 400 mg,
as the trihydrate
CLAVULANIC ACID, 57 mg,
as clavulanate potassium
Rx only 100 mL (when reconstituted)
Use only if inner seal is intact.
Net contents: Equivalent to 8 g amoxicillin and
1.14 g clavulanic acid. Each 5 mL of reconstituted
amoxicillin and clavulanate potassium 400 mg/
57 mg/5 mL oral suspension contains 0.29 mEq
potassium. Store dry powder at 20°-25°C
(68°-77°F) [See USP Controlled Room Temperature].
Directions for mixing: Tap bottle until all powder
flows freely. Add approximately 2/3 of total water for
reconstitution (total = 84 mL); shake vigorously to wet
powder. Add remaining water, again shake vigorously.
Dosage: Administer every 12 hours. See accompanying
prescribing information.
Phenylketonurics: Contains phenylalanine 7 mg per
5 mL. Keep tightly closed. Shake well before using.
Must be refrigerated. Discard after 10 days.
Manufactured for:
Mylan Pharmaceuticals Inc.
Morgantown, WV 26505 U.S.A.
Manufactured in Austria
46179010
RSDZ0834KF1