NDC Code(s) : 0378-3611-01, 0378-3612-01, 0378-3613-01
Packager : Mylan Pharmaceuticals Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Liothyronine sodiumLiothyronine sodium TABLET | ||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Liothyronine sodiumLiothyronine sodium TABLET | ||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Liothyronine sodiumLiothyronine sodium TABLET | ||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 5 mcg
NDC 0378-3611-01
Liothyronine
Sodium
Tablets, USP
5 mcg
Rx only 100 Tablets
Each tablet contains liothyronine
sodium, USP equivalent to 5 mcg
of liothyronine.
Dispense in a tight, light-resistant
container as defined in the USP
using a child-resistant closure.
Keep container tightly closed.
Keep this and all medication
out of the reach of children.
Store at 20° to 25°C (68° to 77°F).
[See USP Controlled Room
Temperature.]
Usual Dosage: See accompanying
prescribing information.
Mylan Pharmaceuticals Inc.
Morgantown, WV 26505 U.S.A.
Mylan.com
RM3611A2

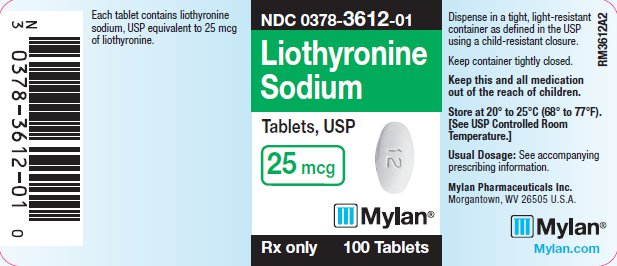
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 25 mcg
NDC 0378-3612-01
Liothyronine
Sodium
Tablets, USP
25 mcg
Rx only 100 Tablets
Each tablet contains liothyronine
sodium, USP equivalent to 25 mcg
of liothyronine.
Dispense in a tight, light-resistant
container as defined in the USP
using a child-resistant closure.
Keep container tightly closed.
Keep this and all medication
out of the reach of children.
Store at 20° to 25°C (68° to 77°F).
[See USP Controlled Room
Temperature.]
Usual Dosage: See accompanying
prescribing information.
Mylan Pharmaceuticals Inc.
Morgantown, WV 26505 U.S.A.
Mylan.com
RM3612A2
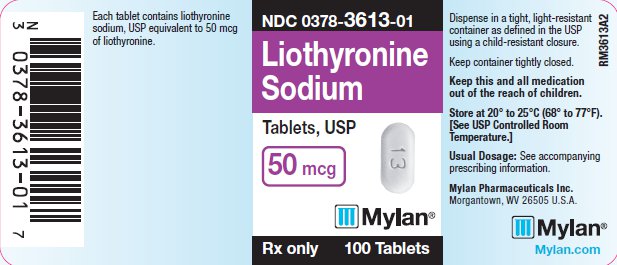
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 50 mcg
NDC 0378-3613-01
Liothyronine
Sodium
Tablets, USP
50 mcg
Rx only 100 Tablets
Each tablet contains liothyronine
sodium, USP equivalent to 50 mcg
of liothyronine.
Dispense in a tight, light-resistant
container as defined in the USP
using a child-resistant closure.
Keep container tightly closed.
Keep this and all medication
out of the reach of children.
Store at 20° to 25°C (68° to 77°F).
[See USP Controlled Room
Temperature.]
Usual Dosage: See accompanying
prescribing information.
Mylan Pharmaceuticals Inc.
Morgantown, WV 26505 U.S.A.
Mylan.com
RM3613A2