NDC Code(s) : 0406-8553-50
Packager : Mallinckrodt, Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : CII
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| OXYCODONE AND ACETAMINOPHENoxycodone and acetaminophen SOLUTION | ||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
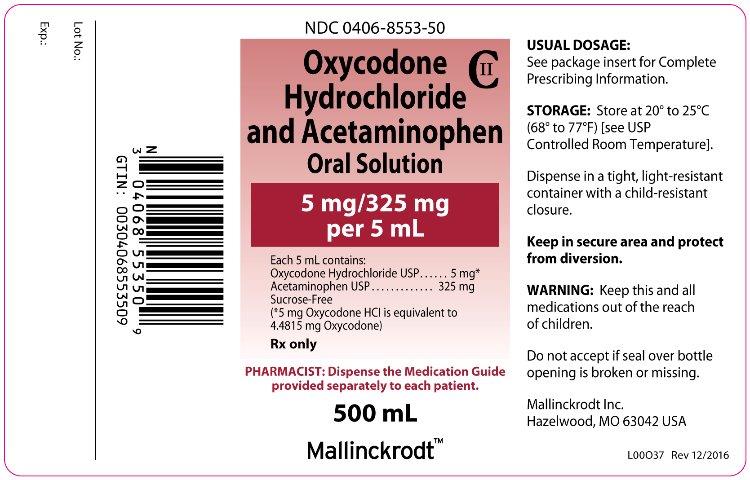
PRINCIPAL DISPLAY PANEL
NDC 0406-8553-50
Oxycodone Hydrochloride and Acetaminophen
Oral Solution
CII
5 mg/325 mg per 5 mL
Each 5 mL contains:
Oxycodone Hydrochloride USP ..... 5mg*
Acetaminophen USP ................. 325 mg
Sucrose-Free
(*5 mg Oxycodone HCl is equivalent to 4.4815 mg Oxycodone)
Rx only
PHARMACIST: Dispense the Medication Guide provided separately to each patient.
500 mL
Mallinckrodt™
L00O37 Rev 12/2016