NDC Code(s) : 0409-1273-03, 0409-1273-32
Packager : Hospira, Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : CIV
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| DiazepamDIAZEPAM INJECTION, SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| LABELER - Hospira, Inc.(141588017) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Hospira, Inc. | 030606222 | ANALYSIS(0409-1273), MANUFACTURE(0409-1273), PACK(0409-1273), LABEL(0409-1273) | |
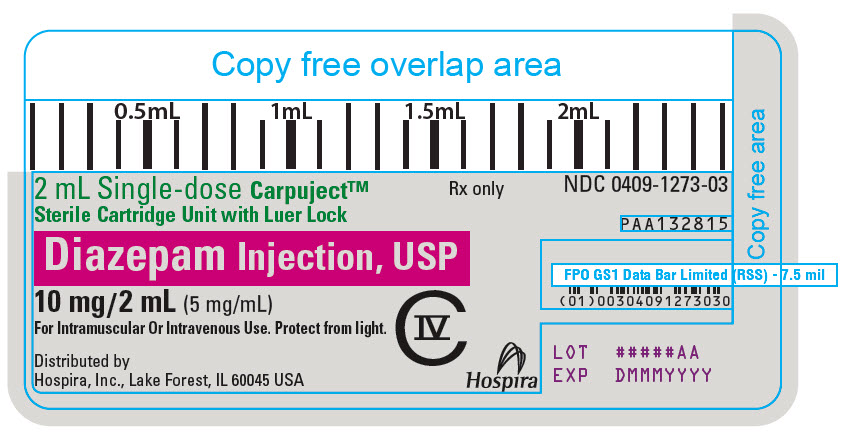
PRINCIPAL DISPLAY PANEL
2 mL Single-dose Carpuject™
Sterile Cartridge Unit with Luer Lock
Rx only
NDC 0409-1273-03
Diazepam Injection, USP
CIV
PAA132815
10 mg/2 mL (5 mg/mL)
For Intramuscular Or Intravenous Use. Protect from light.
Distributed by
Hospira, Inc., Lake Forest, IL 60045 USA
Hospira
LOT #####AA
EXP DMMMYYYY
PRINCIPAL DISPLAY PANEL
NDC 0409-1273-32
Contains 10 of NDC 0409-1273-03
2 mL Single-dose
10 Carpuject™
Sterile Cartridge Units
with Luer Lock
Needle not included
SLIM-PAK™
Tamper Detection Package
Diazepam
Injection, USP
CIV
10 mg/2 mL
(5 mg/mL)
For Intramuscular Or
Intravenous Use
Carpuject Cartridges are to be
used ONLY with Carpuject Holders.
Hospira










