NDC Code(s) : 0409-1934-11, 0409-1934-01, 0409-1933-02, 0409-1933-03
Packager : Hospira, Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Amiodarone HydrochlorideAMIODARONE HYDROCHLORIDE INJECTION, SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Amiodarone HydrochlorideAMIODARONE HYDROCHLORIDE INJECTION, SOLUTION | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
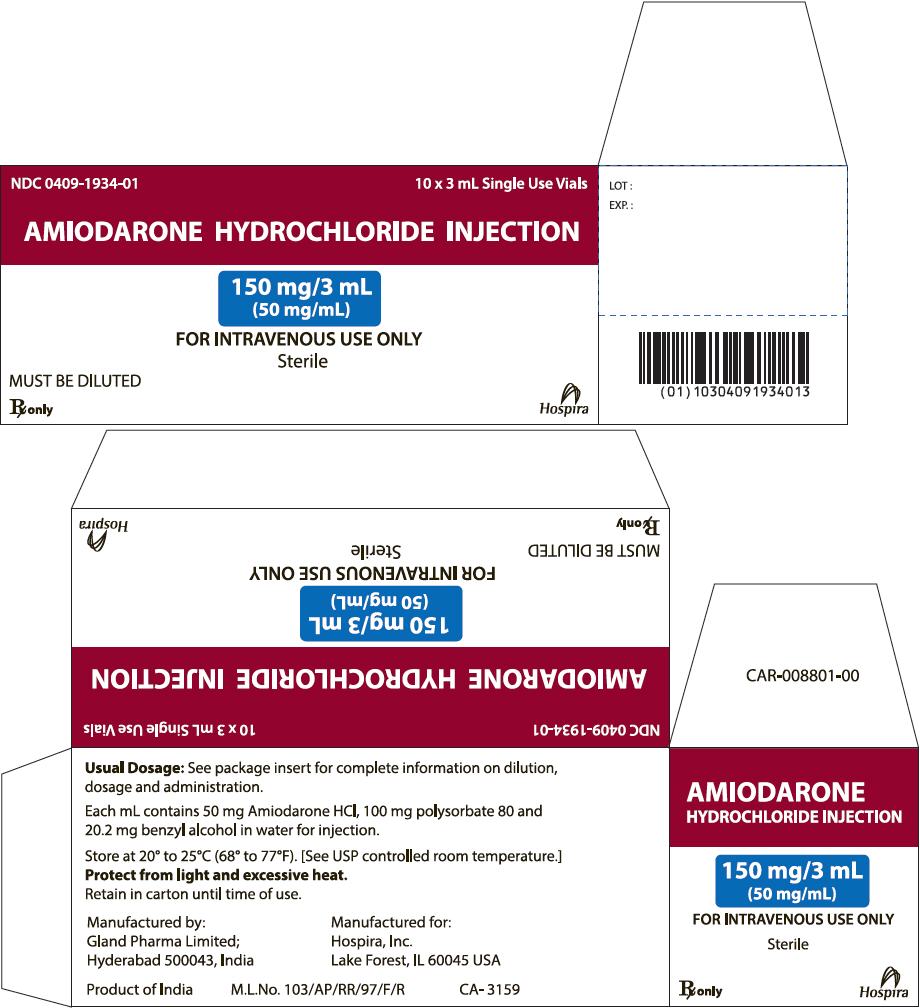
PRINCIPAL DISPLAY PANEL
AMIODARONE
HCl INJECTION
150 mg/3 mL
(50 mg/mL)
FOR INTRAVENOUS USE ONLY
Sterile
MUST BE DILUTED
Rx only
Hospira

PRINCIPAL DISPLAY PANEL
NDC 0409-1934-01
10 x 3 mL Single Use Vials
AMIODARONE HYDROCHLORIDE INJECTION
150 mg/3 mL
(50 mg/mL)
FOR INTRAVENOUS USE ONLY
Sterile
MUST BE DILUTED
Rx only
Hospira
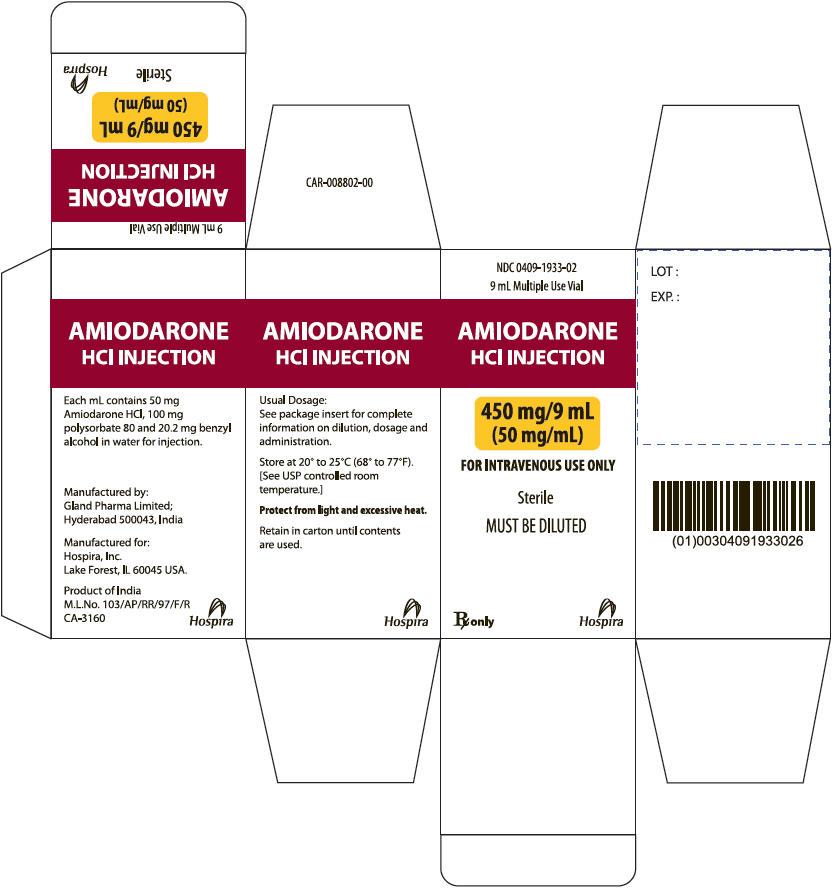
PRINCIPAL DISPLAY PANEL
AMIODARONE
HCl INJECTION
450 mg/9 mL
(50 mg/mL)
FOR INTRAVENOUS USE ONLY
Sterile
MUST BE DILUTED
Rx only
Hospira

PRINCIPAL DISPLAY PANEL
NDC 0409-1933-02
9 mL Multiple Use Vial
AMIODARONE
HCl INJECTION
450 mg/9 mL
(50 mg/mL)
FOR INTRAVENOUS USE ONLY
Sterile
MUST BE DILUTED
Rx only
Hospira
PRINCIPAL DISPLAY PANEL
AMIODARONE
HCl INJECTION
900 mg/18 mL
(50 mg/mL)
FOR INTRAVENOUS USE ONLY
Sterile
MUST BE DILUTED
Rx only
Hospira

PRINCIPAL DISPLAY PANEL
NDC 0409-1933-03
18 mL Multiple Use Vial
AMIODARONE
HCl INJECTION
900 mg/18 mL
(50 mg/mL)
FOR INTRAVENOUS USE ONLY
Sterile
MUST BE DILUTED
Rx only
Hospira










