NDC Code(s) : 0409-1159-18, 0409-1159-01, 0409-1159-19, 0409-1159-02, 0409-1160-18, 0409-1160-01, 0409-1162-18, 0409-1162-01, 0409-1162-19, 0409-1162-02, 0409-1163-18, 0409-1163-01, 0409-1165-18, 0409-1165-01, 0409-1165-19, 0409-1165-02, 0409-9043-11, 0409-9043-01, 0409-9046-11, 0409-9046-01, 0409-9045-11, 0409-9045-01, 0409-9045-16, 0409-9045-17, 0409-9042-11, 0409-9042-01, 0409-9042-16, 0409-9042-17
Packager : Hospira, Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Bupivacaine HydrochlorideBUPIVACAINE HYDROCHLORIDE INJECTION, SOLUTION | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Bupivacaine HydrochlorideBUPIVACAINE HYDROCHLORIDE INJECTION, SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Bupivacaine HydrochlorideBUPIVACAINE HYDROCHLORIDE INJECTION, SOLUTION | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Bupivacaine HydrochlorideBUPIVACAINE HYDROCHLORIDE INJECTION, SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Bupivacaine HydrochlorideBUPIVACAINE HYDROCHLORIDE INJECTION, SOLUTION | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Bupivacaine Hydrochloride and EpinephrineBUPIVACAINE HYDROCHLORIDE AND EPINEPHRINE INJECTION, SOLUTION | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Bupivacaine Hydrochloride and EpinephrineBUPIVACAINE HYDROCHLORIDE AND EPINEPHRINE INJECTION, SOLUTION | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Bupivacaine Hydrochloride and EpinephrineBUPIVACAINE HYDROCHLORIDE AND EPINEPHRINE INJECTION, SOLUTION | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Bupivacaine Hydrochloride and EpinephrineBUPIVACAINE HYDROCHLORIDE AND EPINEPHRINE INJECTION, SOLUTION | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| LABELER - Hospira, Inc.(141588017) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Hospira, Inc. | 093132819 | ANALYSIS(0409-1159, 0409-1160, 0409-1162, 0409-1163, 0409-1165, 0409-9042, 0409-9043, 0409-9045, 0409-9046), MANUFACTURE(0409-1159, 0409-1160, 0409-1162, 0409-1163, 0409-1165, 0409-9042, 0409-9043, 0409-9045, 0409-9046), PACK(0409-1159, 0409-1160, 0409-1162, 0409-1163, 0409-1165, 0409-9042, 0409-9043, 0409-9045, 0409-9046), LABEL(0409-1159, 0409-1160, 0409-1162, 0409-1163, 0409-1165, 0409-9042, 0409-9043, 0409-9045, 0409-9046) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Hospira, Inc. | 827731089 | ANALYSIS(0409-1159, 0409-1160, 0409-1162, 0409-1163, 0409-1165, 0409-9042, 0409-9043, 0409-9045, 0409-9046) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Hospira, Inc. | 030606222 | ANALYSIS(0409-9042, 0409-9043, 0409-9045, 0409-9046) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Pfizer Healthcare India Private Limited | 860037912 | ANALYSIS(0409-1159, 0409-1160, 0409-1162, 0409-1163, 0409-1165), MANUFACTURE(0409-1159, 0409-1160, 0409-1162, 0409-1163, 0409-1165), PACK(0409-1159, 0409-1160, 0409-1162, 0409-1163, 0409-1165), LABEL(0409-1159, 0409-1160, 0409-1162, 0409-1163, 0409-1165) | |
PRINCIPAL DISPLAY PANEL
50 mL
Multiple-dose Fliptop Vial
0.25% Bupivacaine HCl
125 mg/50 mL (2.5 mg/mL)
and epinephrine
1:200,000 Injection
Bupivacaine and Epinephrine Inj., USP
FOR INFILTRATION AND NERVE BLOCK ONLY
Warning: Contains Sulfites
Distributed by Hospira, Inc., Lake Forest, IL 60045 USA
LOT ##-###-AA
EXP DMMMYYYY

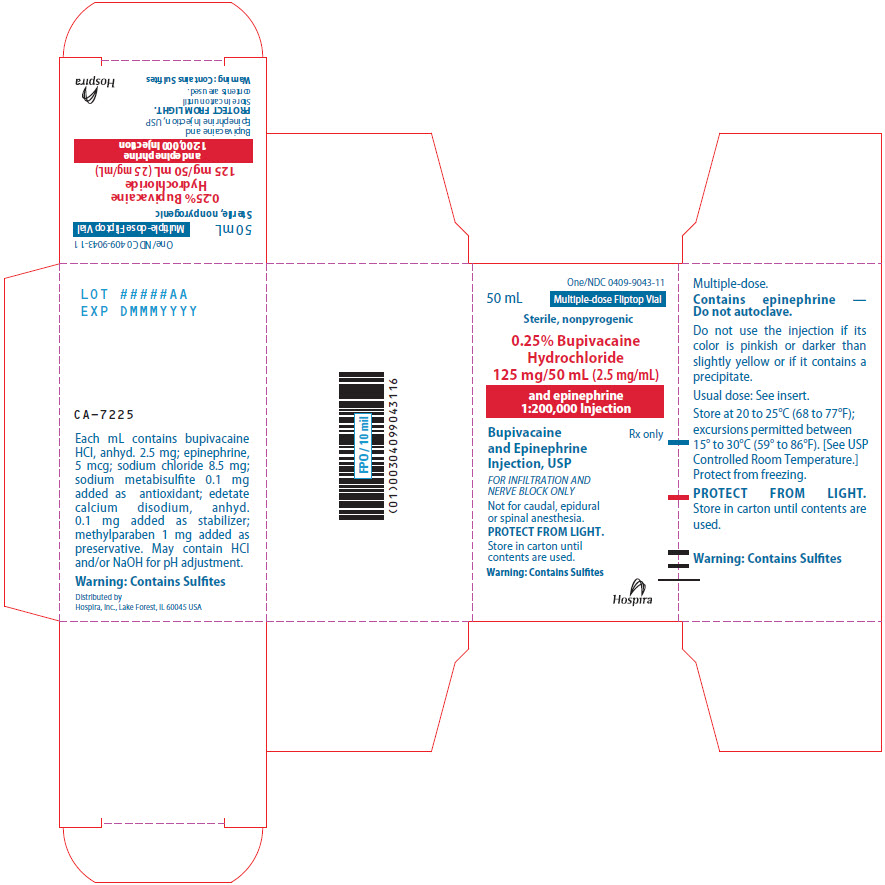
PRINCIPAL DISPLAY PANEL
One/NDC 0409-9043-11
50 mL
Multiple-dose Fliptop Vial
Sterile, nonpyrogenic
0.25% Bupivacaine
Hydrochloride
125 mg/50 mL (2.5 mg/mL)
and epinephrine
1:200,000 Injection
Bupivacaine
and Epinephrine
Injection, USP
Rx only
FOR INFILTRATION AND
NERVE BLOCK ONLY
Not for caudal, epidural
or spinal anesthesia.
PROTECT FROM LIGHT.
Store in carton until
contents are used.
Warning: Contains Sulfites
Hospira
PRINCIPAL DISPLAY PANEL
10 mL Single-dose Teartop Vial
Rx only
0.5% Bupivacaine HCl
50 mg/10 mL (5 mg/mL)
and epinephrine 1:200,000 Injection
Bupivacaine Hydrochloride and
Epinephrine Injection, USP
FOR NERVE BLOCK, CAUDAL
AND EPIDURAL ANESTHESIA ONLY.
Warning: Contains Sulfites
Hospira
Dist. by Hospira, Inc., Lake Forest, IL 60045 USA
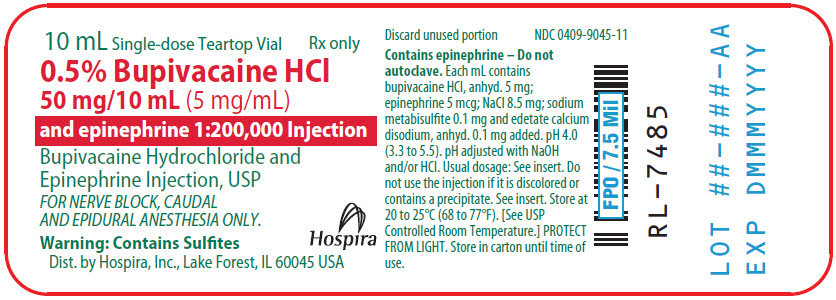
PRINCIPAL DISPLAY PANEL
10 mL Single-dose Teartop Vial
Sterile, Nonpyrogenic
Rx only
NDC 0409-9045-01
Contains 10 of NDC 0409-9045-11
0.5% Bupivacaine Hydrochloride
50 mg/10 mL (5 mg/mL)
and epinephrine 1:200,000 Injection
Bupivacaine Hydrochloride and Epinephrine Injection, USP
FOR NERVE BLOCK, CAUDAL AND EPIDURAL ANESTHESIA ONLY.
Not for spinal anesthesia.
Each mL contains bupivacaine HCl, anhyd. 5 mg; epinephrine 5 mcg; sodium chloride 8.5 mg; sodium metabisulfite 0.1 mg added
as antioxidant; edetate calcium disodium, anhyd. 0.1 mg added as stabilizer. pH 4.0 (3.3 to 5.5). May contain HCl and/or NaOH for
pH adjustment. Contains no antimicrobial preservative. Discard unused portion after initial use.
Warning: Contains Sulfites
Hospira

PRINCIPAL DISPLAY PANEL
50 mL
Multiple-dose Vial
0.5% Bupivacaine HCl
250 mg/50 mL (5 mg/mL)
and epinephrine
1:200,000 Injection
Bupivacaine and Epinephrine Inj., USP
FOR NERVE BLOCK ONLY
Warning: Contains Sulfites
LOT ##-###-AA
EXP DMMMYYYY

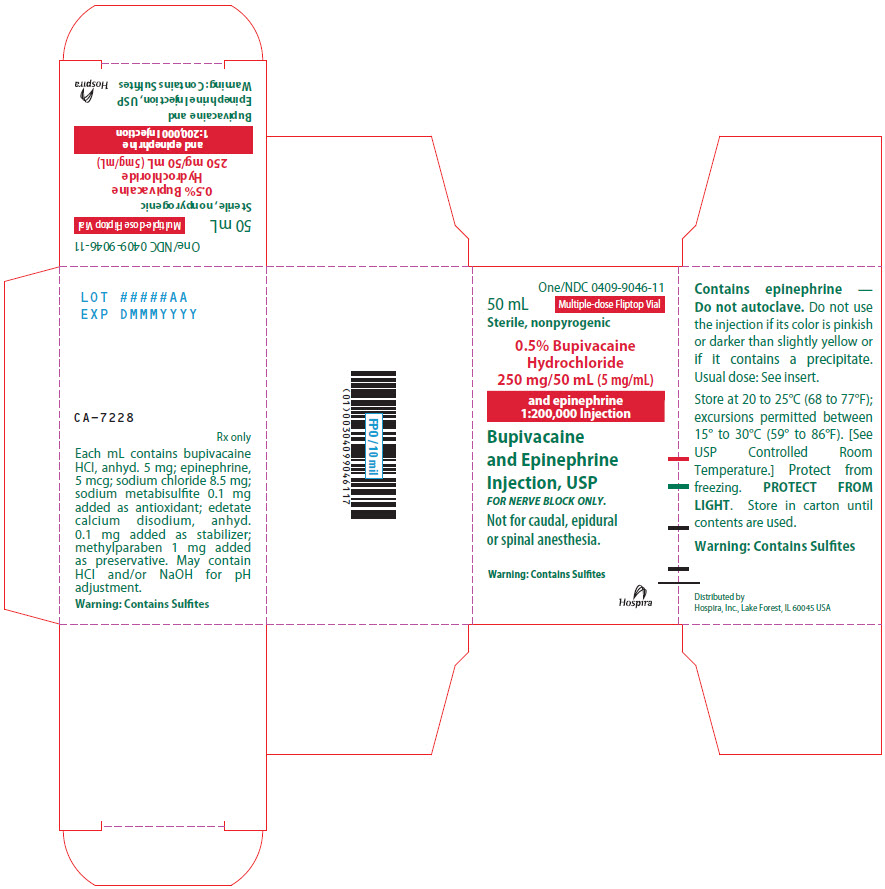
PRINCIPAL DISPLAY PANEL
One/NDC 0409-9046-11
50 mL
Multiple-dose Fliptop Vial
Sterile, nonpyrogenic
0.5% Bupivacaine
Hydrochloride
250 mg/50 mL (5 mg/mL)
and epinephrine
1:200,000 Injection
Bupivacaine
and Epinephrine
Injection, USP
FOR NERVE BLOCK ONLY.
Not for caudal, epidural
or spinal anesthesia.
Warning: Contains Sulfites
Hospira
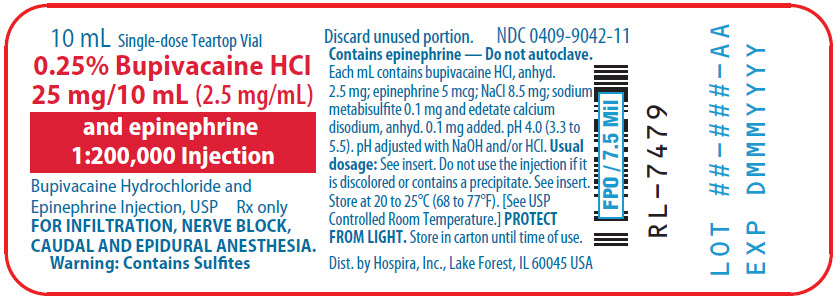
PRINCIPAL DISPLAY PANEL
10 mL Single-dose Teartop Vial
0.25% Bupivacaine HCl
25 mg/10 mL (2.5 mg/mL)
and epinephrine
1:200,000 Injection
Bupivacaine Hydrochloride and
Epinephrine Injection, USP
Rx only
FOR INFILTRATION, NERVE BLOCK,
CAUDAL AND EPIDURAL ANESTHESIA.
Warning: Contains Sulfites
PRINCIPAL DISPLAY PANEL
10 mL
Single-dose
Teartop Vial
Sterile, nonpyrogenic
Rx only
NDC 0409-9042-01
Contains 10 of NDC 0409-9042-11
0.25% Bupivacaine Hydrochloride
25 mg/10 mL (2.5 mg/mL)
and epinephrine 1:200,000 Injection
Bupivacaine Hydrochloride and Epinephrine Injection, USP
FOR INFILTRATION, NERVE BLOCK, CAUDAL AND EPIDURAL ANESTHESIA.
Not for spinal anesthesia.
Each mL contains bupivacaine HCl, anhyd. 2.5 mg; epinephrine 5 mcg; sodium chloride 8.5 mg; sodium
metabisulfite 0.1 mg added as antioxidant; edetate calcium disodium, anhyd. 0.1 mg added as stabilizer.
pH 4.0 (3.3 to 5.5). May contain HCl and/or NaOH for pH adjustment.
Contains no antimicrobial preservative. Discard unused portion after initial use.
Warning: Contains Sulfites
Hospira

PRINCIPAL DISPLAY PANEL
10 mL Single-dose Teartop Vial
Preservative-Free
0.25% Bupivacaine
Hydrochloride Injection, USP
25 mg/10 mL (2.5 mg/mL)
For INFILTRATION, NERVE BLOCK,
CAUDAL and EPIDURAL ANESTHESIA.
Not for intrathecal anesthesia.
Hospira
Dist. by Hospira, Inc., Lake Forest, IL 60045 USA
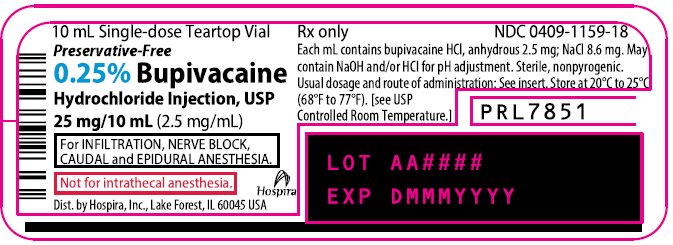
PRINCIPAL DISPLAY PANEL
10 mL Single-dose Teartop Vial
Preservative-Free
Rx only
NDC 0409-1159-01
Contains 25 of NDC 0409-1159-18
0.25% Bupivacaine
Hydrochloride Injection, USP
25 mg/10 mL (2.5 mg/mL)
For INFILTRATION, NERVE BLOCK,
CAUDAL and EPIDURAL ANESTHESIA.
Not for intrathecal anesthesia.
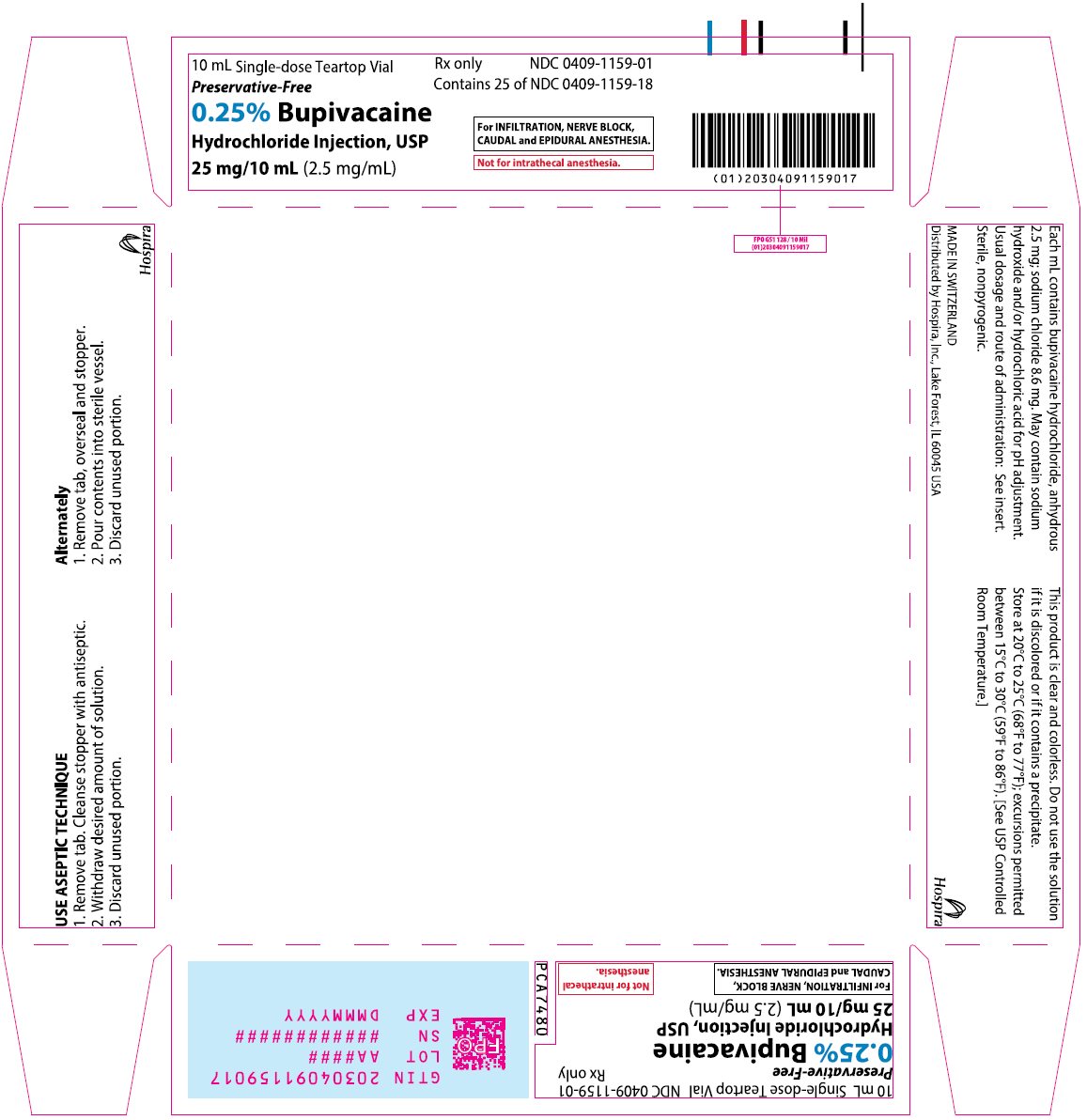
PRINCIPAL DISPLAY PANEL
30 mL Single-dose Teartop Vial
Preservative-Free
0.25% Bupivacaine
Hydrochloride Injection, USP
75 mg/30 mL (2.5 mg/mL)
For INFILTRATION, NERVE BLOCK,
CAUDAL and EPIDURAL ANESTHESIA.
Not for intrathecal anesthesia.
Distributed by Hospira, Inc.
Lake Forest, IL 60045 USA
Hospira
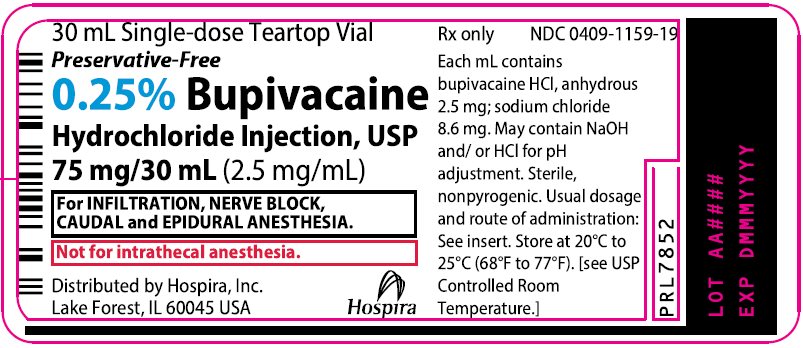
PRINCIPAL DISPLAY PANEL
30 mL Single-dose Teartop Vial
Preservative-Free
Rx only
NDC 0409-1159-02
Contains 25 of NDC 0409-1159-19
0.25% Bupivacaine
Hydrochloride Injection, USP
75 mg/30 mL (2.5 mg/mL)
For INFILTRATION,
NERVE BLOCK, CAUDAL
and EPIDURAL ANESTHESIA.
Not for intrathecal anesthesia.

PRINCIPAL DISPLAY PANEL
50 mL Multiple-dose Fliptop Vial
0.25% Bupivacaine
Hydrochloride Injection, USP
125 mg/50 mL (2.5 mg/mL)
For INFILTRATION and NERVE BLOCK.
Not for caudal, epidural or intrathecal anesthesia.
Distributed by Hospira, Inc.
Lake Forest, IL 60045 USA
Hospira
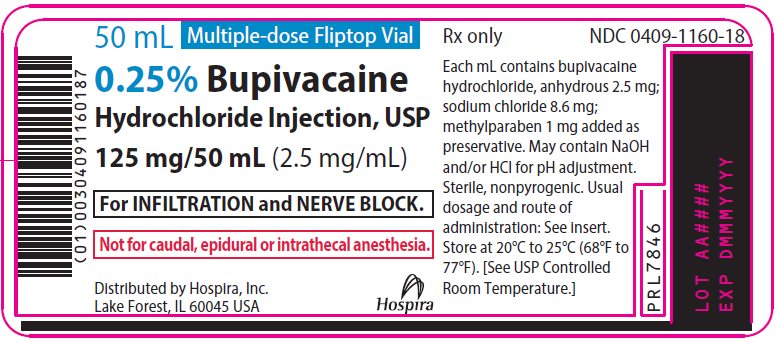
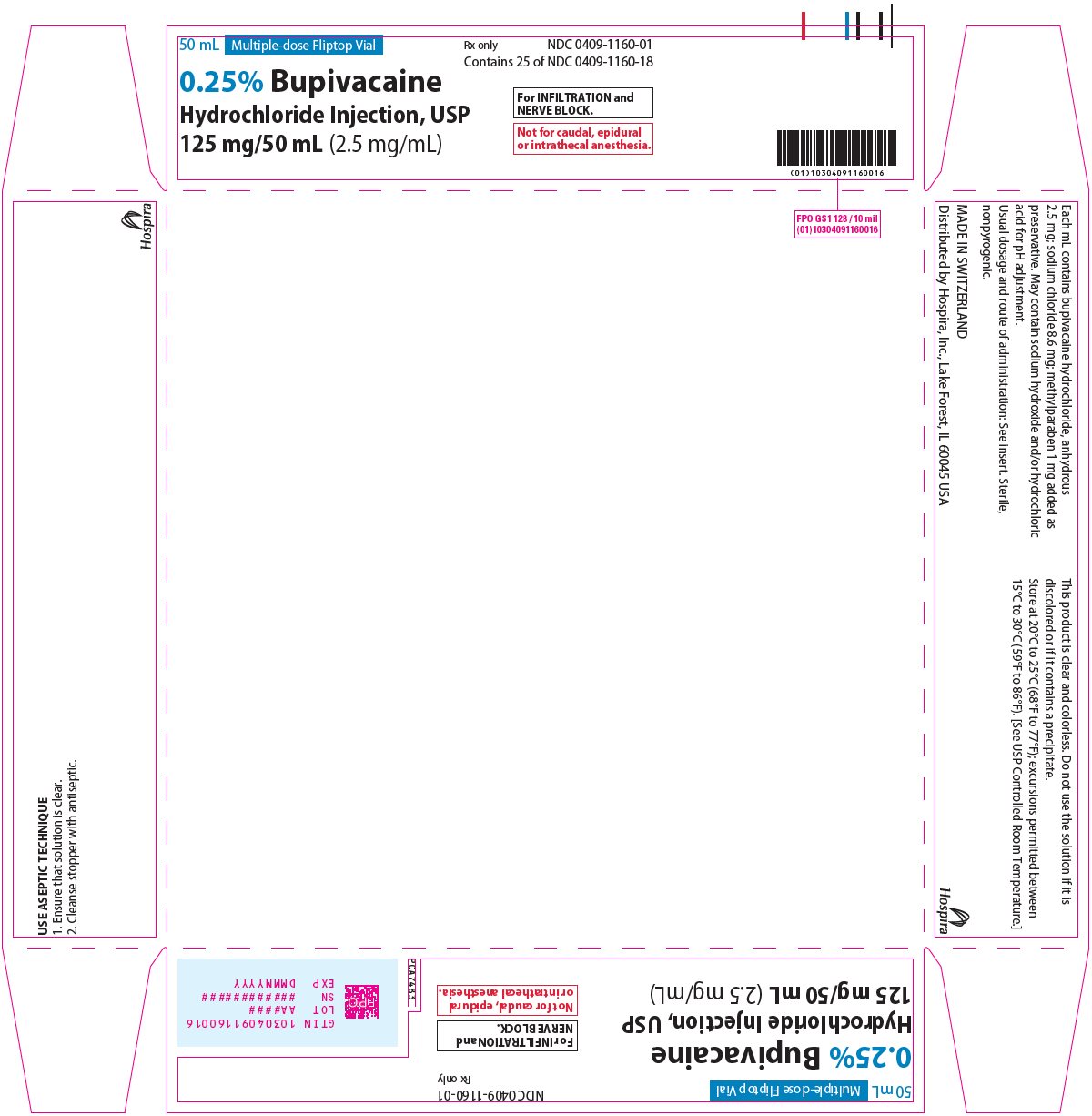
PRINCIPAL DISPLAY PANEL
50 mL Multiple-dose Fliptop Vial
Rx only
NDC 0409-1160-01
Contains 25 of NDC 0409-1160-18
0.25% Bupivacaine
Hydrochloride Injection, USP
125 mg/50 mL (2.5 mg/mL)
For INFILTRATION and
NERVE BLOCK.
Not for caudal, epidural
or intrathecal anesthesia.
PRINCIPAL DISPLAY PANEL
10 mL Single-dose Teartop Vial
Preservative-Free
0.5% Bupivacaine
Hydrochloride Injection, USP
50 mg/10 mL (5 mg/mL)
For NERVE BLOCK, CAUDAL and EPIDURAL ANESTHESIA.
Not for intrathecal anesthesia.
Distributed by Hospira, Inc.
Lake Forest, IL 60045 USA
Hospira
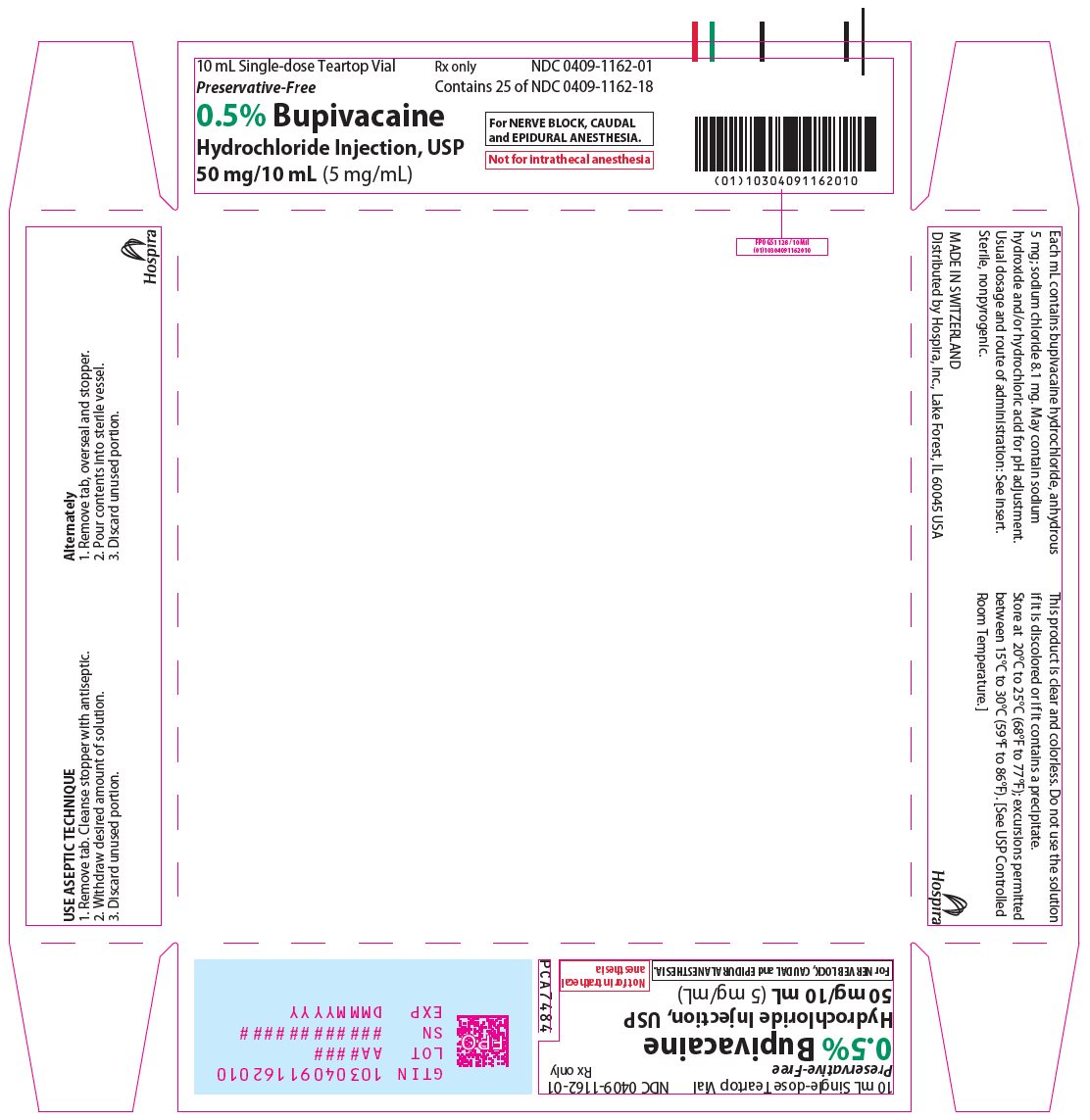
PRINCIPAL DISPLAY PANEL
10 mL Single-dose Teartop Vial
Preservative-Free
Rx only
NDC 0409-1162-01
Contains 25 of NDC 0409-1162-18
0.5% Bupivacaine
Hydrochloride Injection, USP
50 mg/10 mL (5 mg/mL)
For NERVE BLOCK, CAUDAL
and EPIDURAL ANESTHESIA.
Not for intrathecal anesthesia
PRINCIPAL DISPLAY PANEL
30 mL Single-dose Teartop Vial
Preservative-Free
0.5% Bupivacaine
Hydrochloride Injection, USP
150 mg/30 mL (5 mg/mL)
For NERVE BLOCK, CAUDAL and
EPIDURAL ANESTHESIA.
Not for intrathecal anesthesia.
Distributed by Hospira, Inc.
Lake Forest, IL 60045 USA
Hospira
PRINCIPAL DISPLAY PANEL
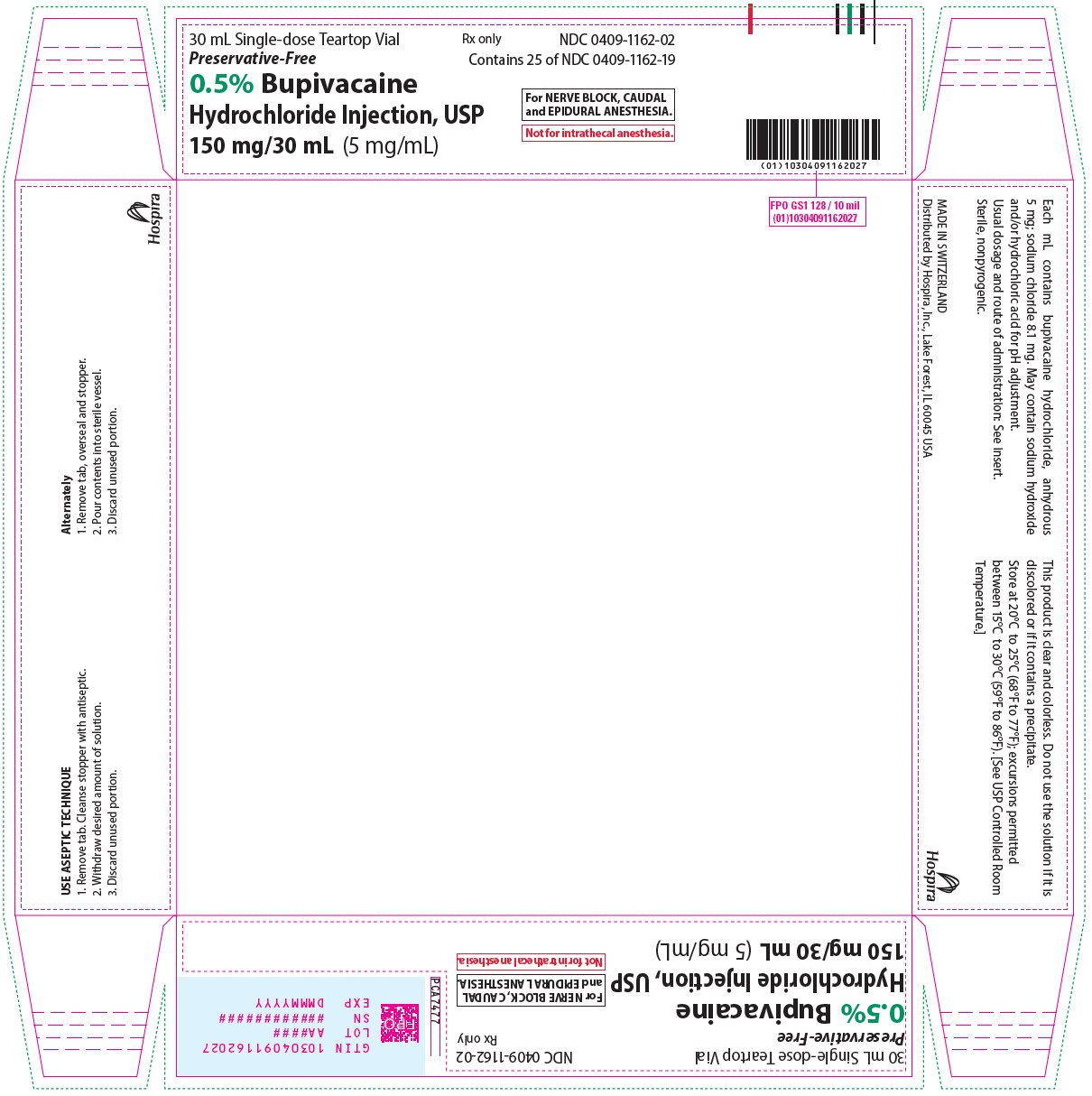
30 mL Single-dose Teartop Vial
Preservative-Free
Rx only
NDC 0409-1162-02
Contains 25 of NDC 0409-1162-19
0.5% Bupivacaine
Hydrochloride Injection, USP
150 mg/30 mL (5 mg/mL)
For NERVE BLOCK, CAUDAL
and EPIDURAL ANESTHESIA.
Not for intrathecal anesthesia.
PRINCIPAL DISPLAY PANEL
50 mL Multiple-dose Fliptop Vial
0.5% Bupivacaine
Hydrochloride Injection, USP
250 mg/50 mL (5 mg/mL)
For NERVE BLOCK.
Not for caudal, epidural or intrathecal anesthesia.
Distributed by Hospira, Inc.,
Lake Forest, IL 60045 USA
Hospira
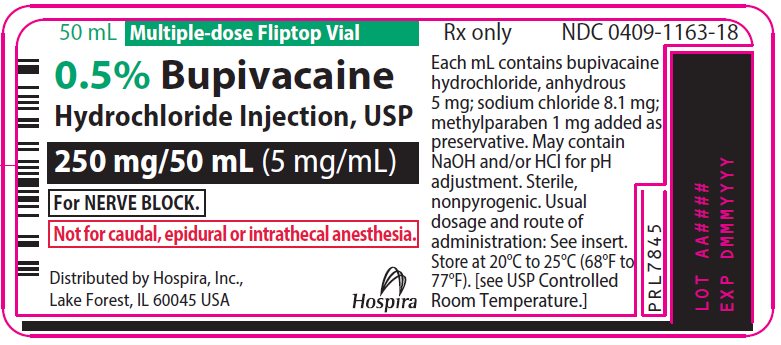
PRINCIPAL DISPLAY PANEL
50 mL Multiple-dose Fliptop Vial
Rx only
NDC 0409-1163-01
Contains 25 of NDC 0409-1163-18
0.5% Bupivacaine
Hydrochloride Injection, USP
250 mg/50 mL (5 mg/mL)
For NERVE BLOCK.
Not for caudal, epidural
or intrathecal anesthesia.
PRINCIPAL DISPLAY PANEL
10 mL Single-dose Teartop Vial
0.75%
Bupivacaine
Hydrochloride Injection, USP
75 mg/10 mL (7.5 mg/mL)
For EPIDURAL and RETROBULBAR ANESTHESIA.
Not for OBSTETRIC or INTRATHECAL ANESTHESIA.
Dist. by Hospira, Inc. Lake Forest, IL 60045 USA

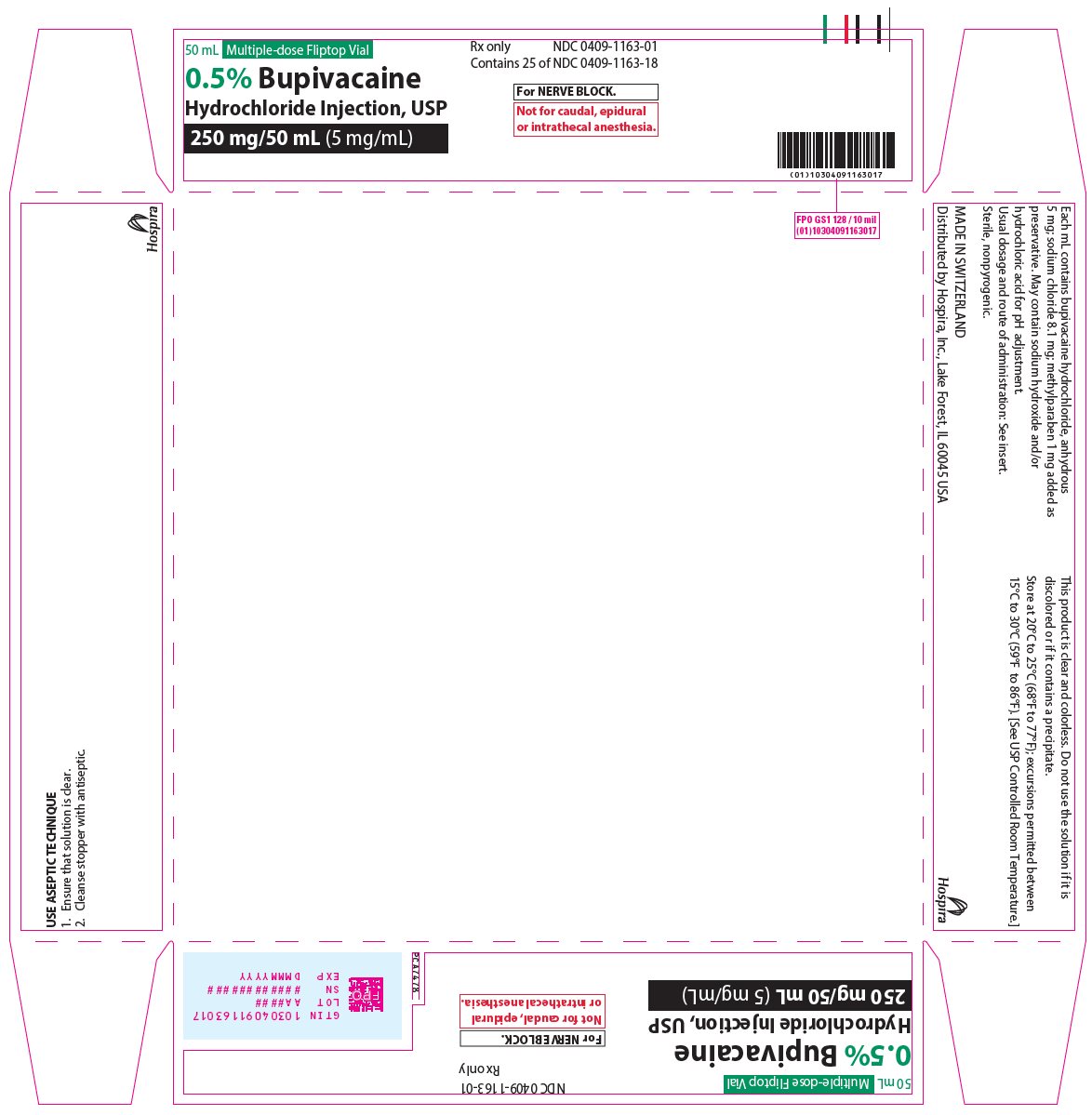
PRINCIPAL DISPLAY PANEL
10 mL Single-dose Teartop Vial
Preservative-Free
Rx only
NDC 0409-1165-01
Contains 25 of NDC 0409-1165-18
0.75% Bupivacaine
Hydrochloride Injection, USP
75 mg/10 mL (7.5 mg/mL)
For EPIDURAL and
RETROBULBAR ANESTHESIA.
Not for OBSTETRIC or
INTRATHECAL ANESTHESIA.
PRINCIPAL DISPLAY PANEL
30 mL Single-dose Teartop Vial
Preservative-Free
0.75% Bupivacaine
Hydrochloride Injection, USP
225 mg/30 mL (7.5 mg/mL)
For EPIDURAL and RETROBULBAR ANESTHESIA.
Not for OBSTETRIC or INTRATHECAL ANESTHESIA.
Distributed by Hospira, Inc.,
Lake Forest, IL 60045 USA
Hospira
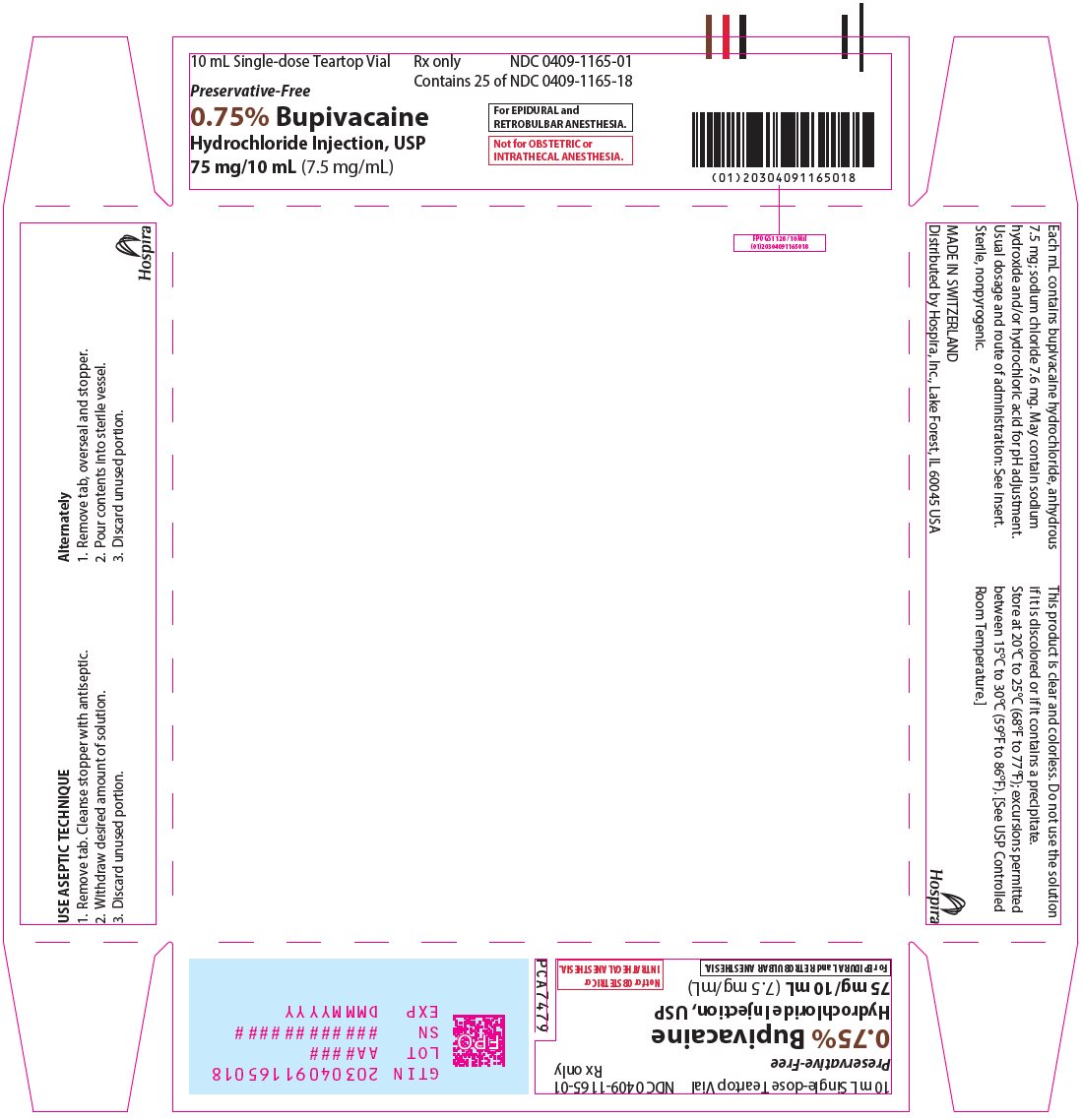
PRINCIPAL DISPLAY PANEL
30 mL Single-dose Teartop Vial
Preservative-Free
Rx only
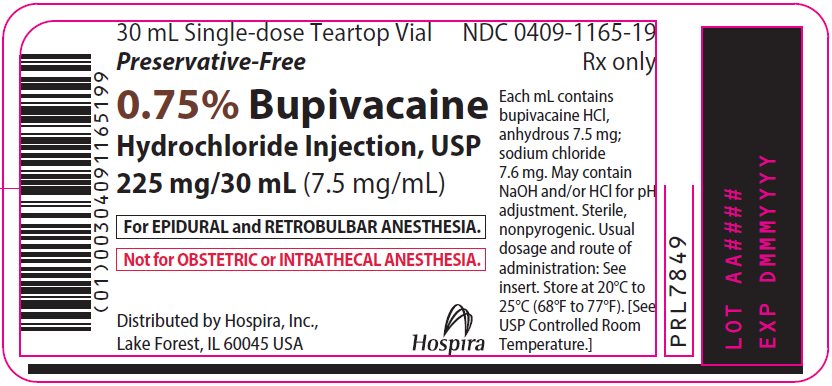
NDC 0409-1165-02
Contains 25 of NDC 0409-1165-19
0.75% Bupivacaine
Hydrochloride Injection, USP
225 mg/30 mL (7.5 mg/mL)
For EPIDURAL and RETROBULBAR ANESTHESIA.
Not for OBSTETRIC or INTRATHECAL ANESTHESIA.

PRINCIPAL DISPLAY PANEL
30 mL Single-dose Teartop Vial
Rx only
0.25% Bupivacaine HCl
75 mg/30 mL (2.5 mg/mL)
and epinephrine
1:200,000 Injection
Bupivacaine Hydrochloride
and Epinephrine Injection, USP
FOR INFILTRATION, NERVE BLOCK,
CAUDAL AND EPIDURAL ANESTHESIA.
Warning: Contains Sulfites
LOT ##-###-AA
EXP DMMMYYYY

PRINCIPAL DISPLAY PANEL
30 mL Single-dose Teartop Vial
Rx only
NDC 0409-9042-17
Contains 25 of NDC 0409-9042-16
0.25% Bupivacaine Hydrochloride
75 mg/30 mL (2.5 mg/mL)
and epinephrine 1:200,000 Injection
Sterile, nonpyrogenic
Bupivacaine Hydrochloride and Epinephrine Injection, USP
FOR INFILTRATION, NERVE BLOCK, CAUDAL AND EPIDURAL ANESTHESIA.
Not for spinal anesthesia.
Warning: Contains Sulfites
Hospira

PRINCIPAL DISPLAY PANEL
30 mL Single-dose Teartop Vial
Discard unused portion
0.5% Bupivacaine HCl
150 mg/30 mL (5 mg/mL)
and epinephrine
1:200,000 Injection
Bupivacaine Hydrochloride and
Epinephrine Injection, USP
FOR NERVE BLOCK, CAUDAL
AND EPIDURAL ANESTHESIA ONLY.
Warning: Contains Sulfites
PRINCIPAL DISPLAY PANEL
30 mL Single-dose Teartop Vial
Sterile, Nonpyrogenic
Rx only
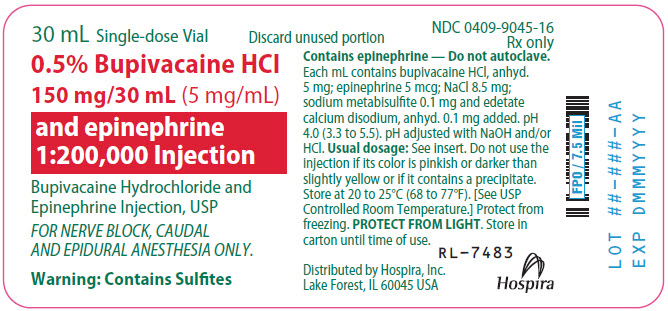
NDC 0409-9045-17
Contains 25 of NDC 0409-9045-16
0.5% Bupivacaine Hydrochloride
150 mg/30 mL (5 mg/mL)
and epinephrine 1:200,000 Injection
Bupivacaine Hydrochloride and Epinephrine Injection, USP
FOR NERVE BLOCK, CAUDAL AND EPIDURAL ANESTHESIA ONLY.
Not for spinal anesthesia.
Warning: Contains Sulfites










