NDC Code(s) : 0430-0836-95, 0430-0836-20, 0430-0838-95, 0430-0838-19
Packager : Allergan, Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| DORYXdoxycycline hyclate CAPSULE, DELAYED RELEASE PELLETS | |||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
| DORYXdoxycycline hyclate CAPSULE, DELAYED RELEASE PELLETS | |||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
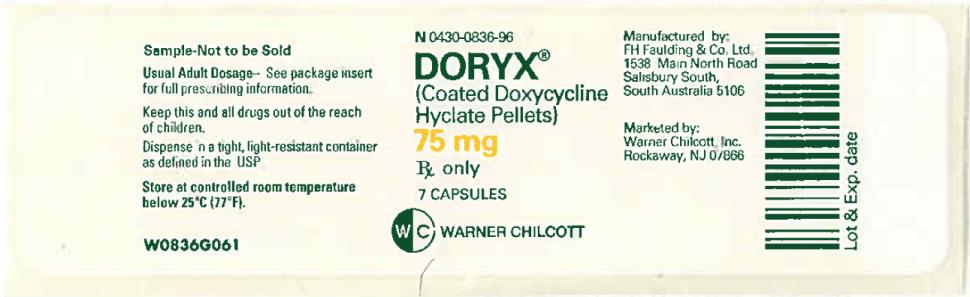
PRINCIPAL DISPLAY PANEL
NDC 0430-0836-96
DORYX
(Coated Doxycycline
Hyclate Pellets)
75 mg
7 Capsules
Rx Only
PRINCIPAL DISPLAY PANEL
NDC 0430-0836-20
DORYX
(Coated Doxycycline
Hyclate Pellets)
75 mg
60 Capsules
Rx Only

PRINCIPAL DISPLAY PANEL
NDC 0430-0838-96
DORYX
(Coated Doxycycline
Hyclate Pellets)
100 mg
7 Capsules
Rx Only

PRINCIPAL DISPLAY PANEL
NDC 0430-0838-19
DORYX
(Coated Doxycycline
Hyclate Pellets)
100 mg
50 Capsules
Rx Only










