NDC Code(s) : 0456-0457-01, 0456-1045-01, 0456-0458-01, 0456-0458-11, 0456-0458-63, 0456-0459-01, 0456-0459-11, 0456-0459-63, 0456-0460-01, 0456-0461-01, 0456-0461-11, 0456-0461-63, 0456-0462-01, 0456-0463-01, 0456-0464-01
Packager : Allergan, Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Armour ThyroidTHYROID, PORCINE TABLET | ||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Armour ThyroidTHYROID, PORCINE TABLET | ||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Armour ThyroidTHYROID, PORCINE TABLET | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Armour ThyroidTHYROID, PORCINE TABLET | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Armour ThyroidTHYROID, PORCINE TABLET | ||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Armour ThyroidTHYROID, PORCINE TABLET | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Armour ThyroidTHYROID, PORCINE TABLET | ||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Armour ThyroidTHYROID, PORCINE TABLET | ||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Armour ThyroidTHYROID, PORCINE TABLET | ||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| LABELER - Allergan, Inc.(144796497) |
PRINCIPAL DISPLAY PANEL
NDC 0456-0457-01
Armour ® Thyroid
(thyroid tablets, USP)
¼ GRAIN (15 mg)
Each tablet contains:
levothyroxine (T4) 9.5 mcg
liothyronine (T3) 2.25 mcg
100 TABLETS
abbvie
Rx only

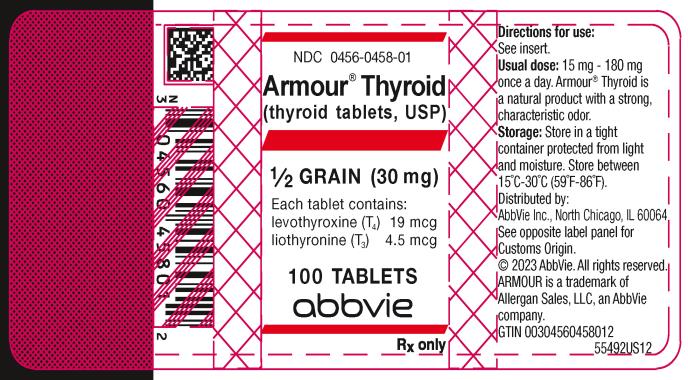
PRINCIPAL DISPLAY PANEL
NDC 0456-0458-01
Armour ® Thyroid
(thyroid tablets, USP)
½ GRAIN (30 mg)
Each tablet contains:
levothyroxine (T4) 19 mcg
liothyronine (T3) 4.5 mcg
100 TABLETS
abbvie
Rx only
PRINCIPAL DISPLAY PANEL
NDC 0456-0459-01
Armour ® Thyroid
(thyroid tablets, USP)
1 GRAIN (60 mg)
Each tablet contains:
levothyroxine (T4) 38 mcg
liothyronine (T3) 9 mcg
100 TABLETS
abbvie
Rx only

PRINCIPAL DISPLAY PANEL
NDC 0456-0460-01
Armour ® Thyroid
(thyroid tablets, USP)
1½ GRAIN (90 mg)
Each tablet contains:
levothyroxine (T4) 57 mcg
liothyronine (T3) 13.5 mcg
100 TABLETS
abbvie
Rx only

PRINCIPAL DISPLAY PANEL
NDC 0456-0461-01
Armour ® Thyroid
(thyroid tablets, USP)
2 GRAIN (120 mg)
Each tablet contains:
levothyroxine (T4) 76 mcg
liothyronine (T3) 18 mcg
100 TABLETS
abbvie
Rx only

PRINCIPAL DISPLAY PANEL
NDC 0456-0462-01
Armour ® Thyroid
(thyroid tablets, USP)
3 GRAIN (180 mg)
Each tablet contains:
levothyroxine (T4) 114 mcg
liothyronine (T3) 27 mcg
100 TABLETS
abbvie
Rx only

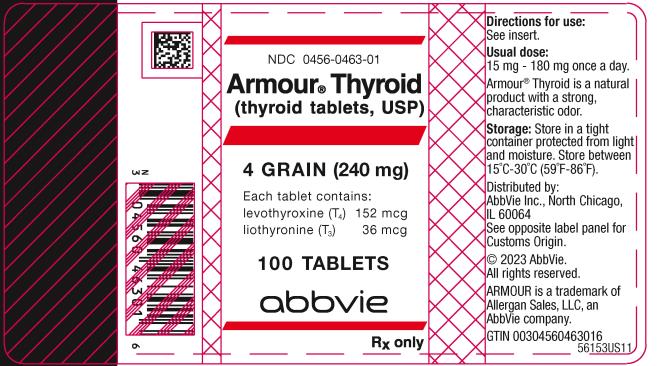
PRINCIPAL DISPLAY PANEL
NDC 0456-0463-01
Armour ® Thyroid
(thyroid tablets, USP)
4 GRAIN (240 mg)
Each tablet contains:
levothyroxine (T4) 152 mcg
liothyronine (T3) 36 mcg
100 TABLETS
abbvie
Rx only
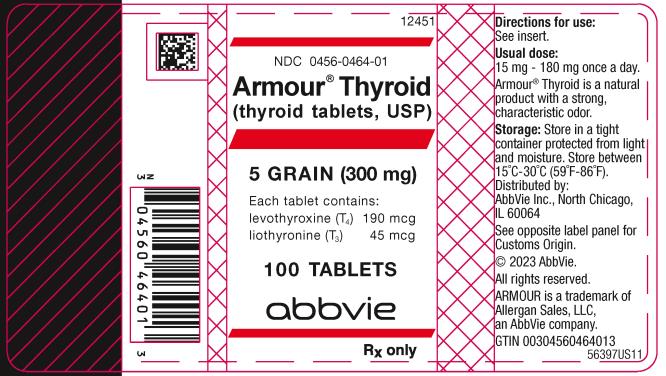
PRINCIPAL DISPLAY PANEL
NDC 0456-0464-01
Armour ® Thyroid
(thyroid tablets, USP)
5 GRAIN (300 mg)
Each tablet contains:
levothyroxine (T4) 190 mcg
liothyronine (T3) 45 mcg
100 TABLETS
abbvie
Rx only
PRINCIPAL DISPLAY PANEL
NDC 0456-1045-01
Armour ® Thyroid
(thyroid tablets, USP)
1/4 GRAIN ( 15 mg)










