NDC Code(s) : 0472-0804-15, 0472-0804-60
Packager : Actavis Mid Atlantic LLC
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| DesonideDesonide CREAM | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
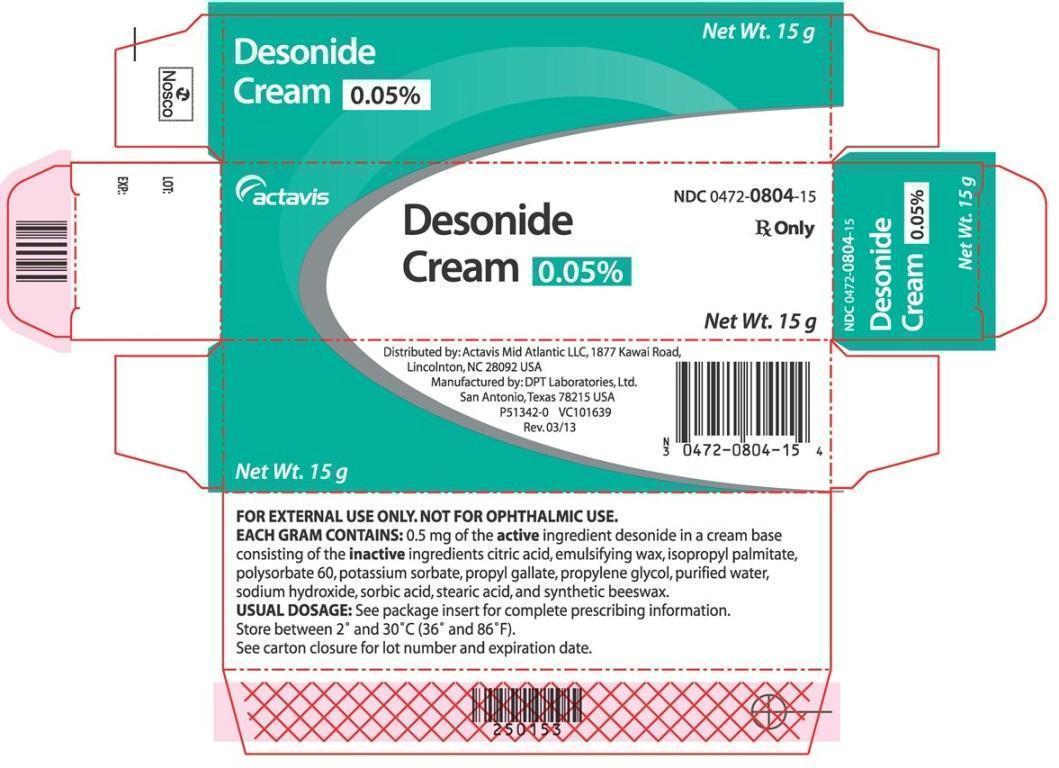
PRINCIPAL DISPLAY PANEL
Nosco
Desonide Cream 0.05% Net Wt. 15 g
LOT:
EXP:
actavis NDC 0472-0804-15
Desonide Cream 0.05% Rx Only
Net Wt. 15 g
NDC 0472-0804-15
Desonide Cream 0.05% Net Wt. 15 g
Distributed by: Actavis Mid Atlantic LLC, 1877 Kawai Road,
Lincolnton, NC 28092 USA
Manufactured by: DPT Laboratories, Ltd.
San Antonio, Texas 78215 USA
P51342-0 VC101639
Rev. 03/13
Net Wt. 15 g
FOR EXTERNAL USE ONLY. NOT FOR OPHTHALMIC USE.
EACH GRAM CONTAINS: 0.5 mg of the active ingredient desonide in a cream base consisting of the inactive ingredients citric acid, emulsifying wax, isopropyl palmitate, polysorbate 60, potassium sorbate, propyl gallate, propylene glycol, purified water, sodium hydroxide, sorbic acid, and synthetic beeswax.
USUAL DOSAGE: See package insert for complete prescribing information.
Store between 2° and 30°C (36° and 86°F).
See carton closure for lot number and expiration date.









