NDC Code(s) : 0472-1202-06, 0472-1202-50
Packager : Actavis Mid Atlantic LLC
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| FeverALL Junioracetaminophen SUPPOSITORY | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
PRINCIPAL DISPLAY PANEL
Jr. Strength
Ages 6-12 years
325 mg
NDC 0472-1202-06
FeverAll®
Acetaminophen Suppositories
Pain reliever/fever reducer
6 Rectal Suppositories
325 mg each
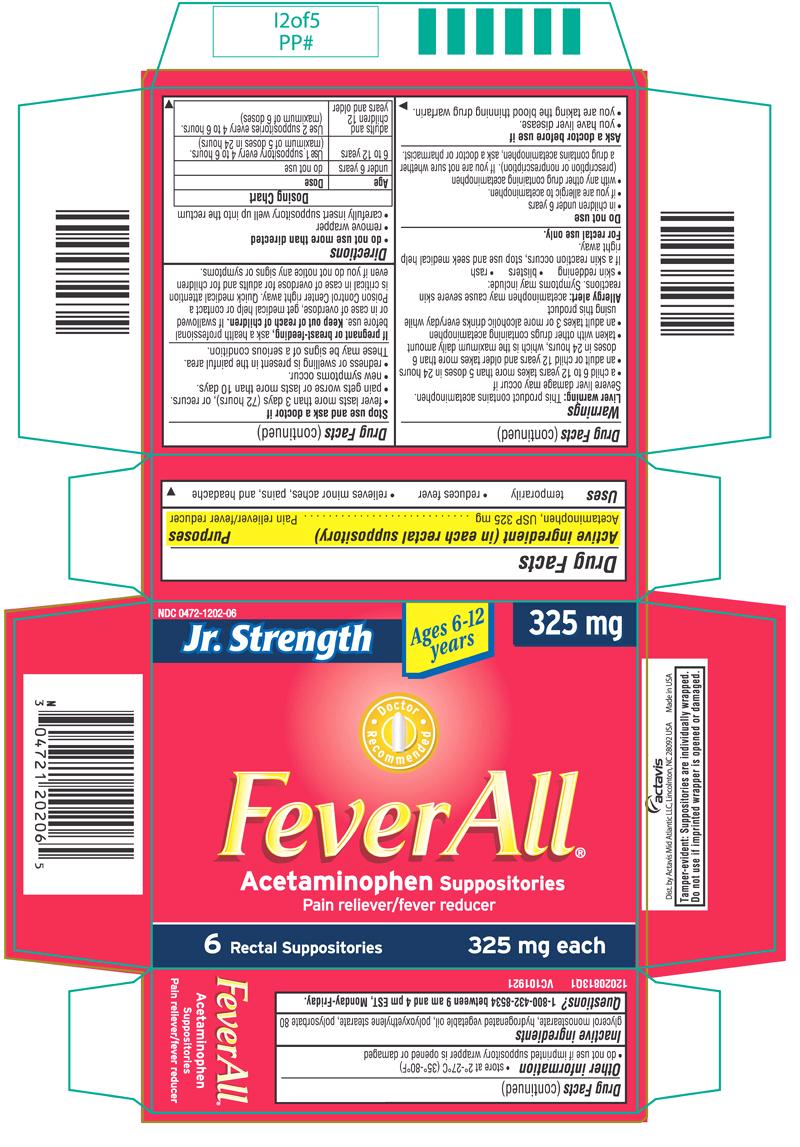 6 RECTAL SUPPOSITORIES CARTON LABEL
6 RECTAL SUPPOSITORIES CARTON LABEL









