NDC Code(s) : 0472-1203-50
Packager : Actavis Mid Atlantic LLC
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| FeverALL Adultsacetaminophen SUPPOSITORY | |||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
PRINCIPAL DISPLAY PANEL
Adults’
Ages 12 Years And Older
NOT FOR RETAIL SALE
NDC 0472-1203-50
FeverAll ®
Acetaminophen Suppositories
Pain reliever/fever reducer
50 Rectal Suppositories
650 mg each
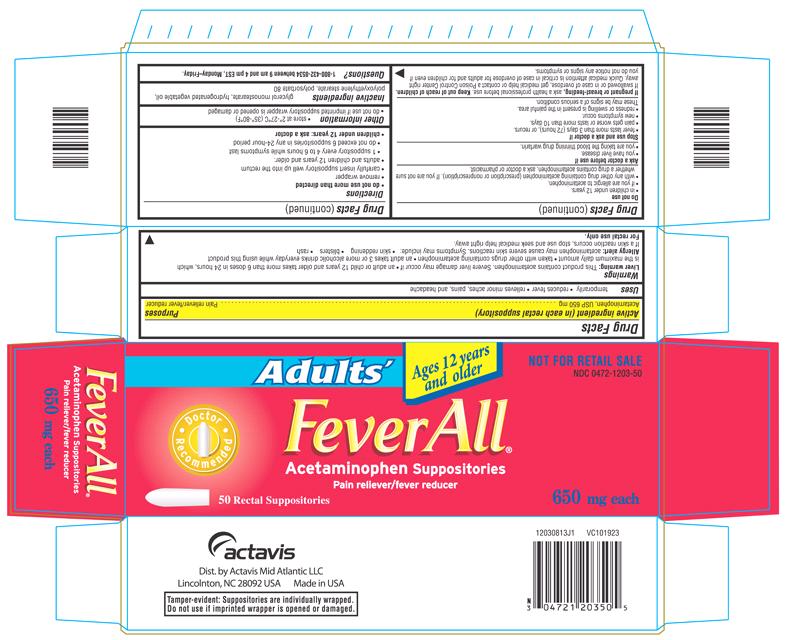 50 RECTAL SUPPOSITORIES CARTON LABEL
50 RECTAL SUPPOSITORIES CARTON LABEL









