NDC Code(s) : 0517-0134-10, 0517-0234-10
Packager : American Regent, Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Dexferrumiron dextran INJECTION, SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Dexferrumiron dextran INJECTION, SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
PRINCIPAL DISPLAY PANEL
CONTAINER
NDC 0517-0134-10
DEXFERRUM® (IRON DEXTRAN INJECTION, USP)
50 mg Elemental Iron per mL
1 mL SINGLE DOSE VIAL
FOR IV USE
Rx Only
AMERICAN REGENT, INC.
SHIRLEY, NY 11967

CARTON
NDC 0517-0134-10
10 x 1 mL SINGLE DOSE VIALS
DEXFERRUM® (IRON DEXTRAN INJECTION, USP)
50 mg Elemental Iron per mL
1 mL SINGLE DOSE VIAL
FOR INTRAVENOUS USE
Rx Only
AMERICAN REGENT, INC.
SHIRLEY, NY 11967
U.S. PATENT 5,624,668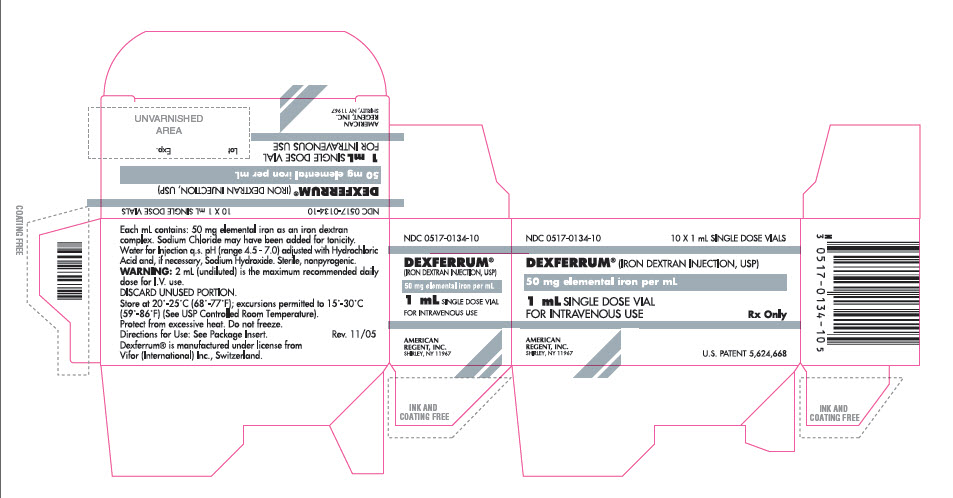
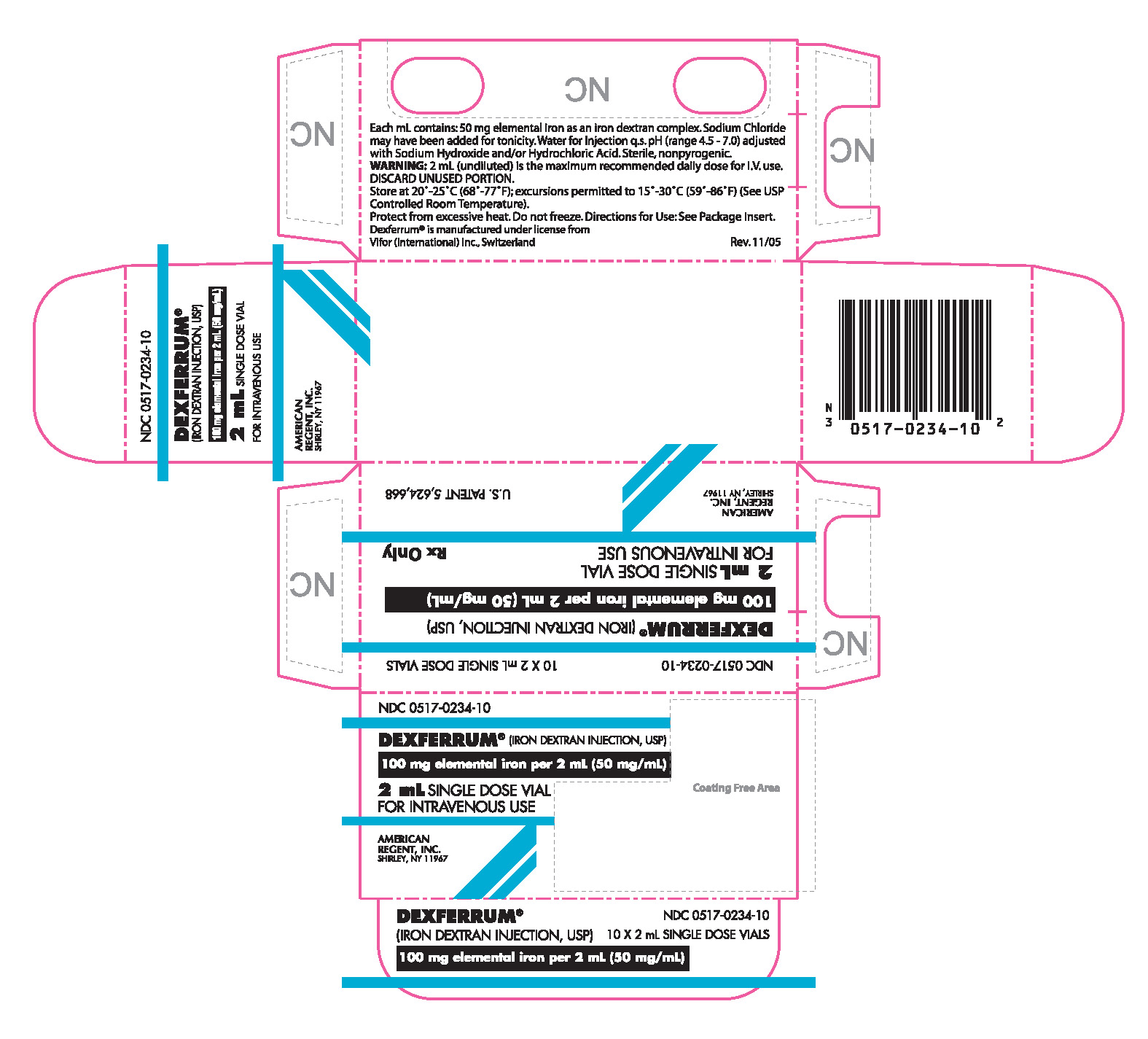
PRINCIPAL DISPLAY PANEL
CONTAINER
NDC 0517-0234-10
DEXFERRUM® (IRON DEXTRAN INJECTION, USP)
100 mg Elemental Iron per 2 mL (50 mg/mL)
2 mL SINGLE DOSE VIAL
FOR IV USE
Rx Only
AMERICAN REGENT, INC.
SHIRLEY, NY 11967

CARTON
NDC 0517-0234-10
10 x 2 mL SINGLE DOSE VIALS
DEXFERRUM® (IRON DEXTRAN INJECTION, USP)
100 mg Elemental Iron per 2 mL (50 mg/mL)
2 mL SINGLE DOSE VIAL
FOR INTRAVENOUS USE
Rx Only
AMERICAN REGENT, INC.
SHIRLEY, NY 11967
U.S. PATENT 5,624,668