NDC Code(s) : 0519-1381-80, 0519-1381-90, 0519-1381-36, 0519-1381-35
Packager : STERIS Corporation
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Soft N Sure Foamed Antiseptic HandrubALCOHOL AEROSOL, FOAM | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
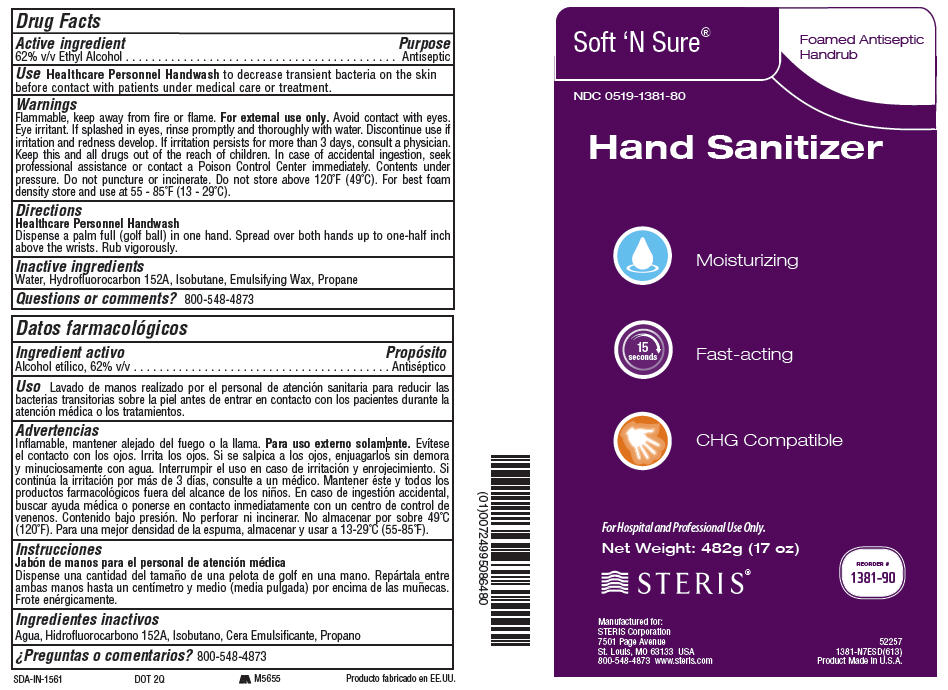
PRINCIPAL DISPLAY PANEL
Soft 'N Sure®
Foamed Antiseptic
Handrub
NDC 0519-1381-80
Hand Sanitizer
Moisturizing
15
seconds
Fast-acting
CHG Compatible
For Hospital and Professional Use Only.
Net Weight: 482g (17 oz)
STERIS®
REORDER #
1381-90
Manufactured for:
STERIS Corporation
7501 Page Avenue
St. Louis, MO 63133 USA
800-548-4873 www.steris.com
52257
1381-N7ESD(613)
Product Made in U.S.A.