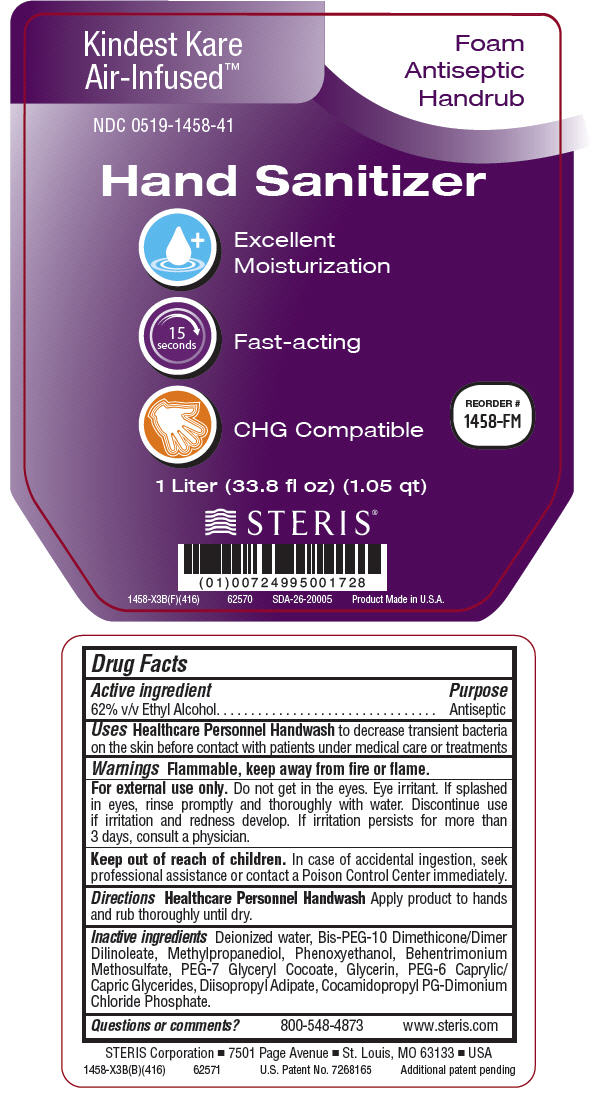
NDC Code(s) : 0519-1458-41, 0519-1458-87, 0519-1458-13, 0519-1458-91
Packager : STERIS Corporation
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Kindest Kare Air-Infused Foam Antiseptic HandrubALCOHOL LIQUID | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
PRINCIPAL DISPLAY PANEL
Kindest Kare
Air-Infused™
Foam
Antiseptic
Handrub
NDC 0519-1458-41
Hand Sanitizer
Excellent
Moisturization
15
seconds
Fast-acting
CHG Compatible
REORDER #
1458-FM
1 Liter (33.8 fl oz) (1.05 qt)
STERIS ®
1458-X3B(F)(416)
62570
SDA-26-20005
Product Made in U.S.A.