NDC Code(s) : 0555-1054-60, 0555-1054-86, 0555-1054-56, 0555-1055-60, 0555-1055-86, 0555-1055-56, 0555-1056-60, 0555-1056-86, 0555-1057-60, 0555-1057-56, 0555-1057-86
Packager : Teva Pharmaceuticals USA, Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| ClaravisIsotretinoin CAPSULE, LIQUID FILLED | ||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| ClaravisIsotretinoin CAPSULE, LIQUID FILLED | ||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
| ClaravisIsotretinoin CAPSULE, LIQUID FILLED | ||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| ClaravisIsotretinoin CAPSULE, LIQUID FILLED | ||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| LABELER - Teva Pharmaceuticals USA, Inc.(001627975) |
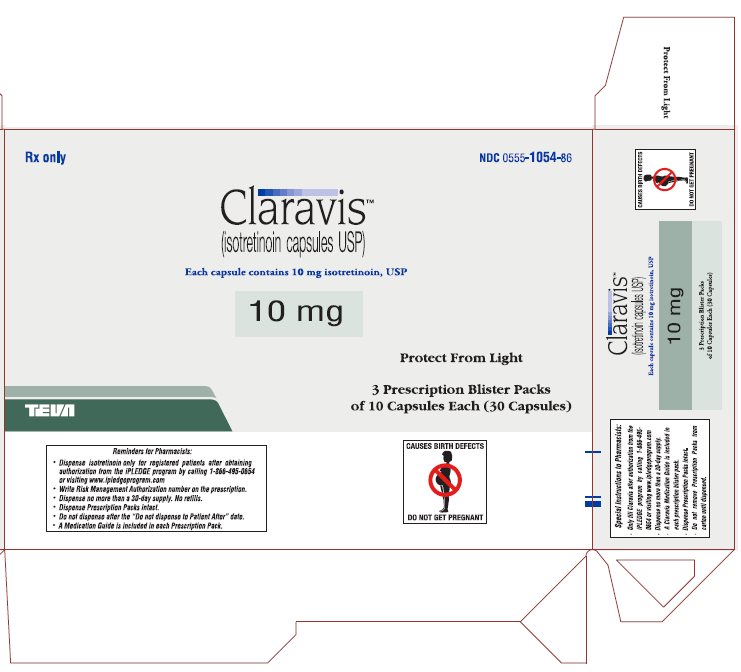
PRINCIPAL DISPLAY PANEL
Rx only
NDC 0555-1054-86
ClaravisTM
(isotretinoin capsules USP)
Each capsule contains 10 mg isotretinoin, USP
10 mg
Protect from Light
3 Prescription Blister Packs
of 10 Capsules Each (30 Capsules)
CONTRAINDICATED IN PREGNANCY
PHARMACIST: DISPENSE PRESCRIPTION BLISTER PACKS INTACT.
Blister pack complies with child-resistant packaging requirements.
Usual Dosage: See package brochure and other important prescribing information.
Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature].
TEVA PHARMACEUTICALS USA, Inc., North Wales, PA 19454
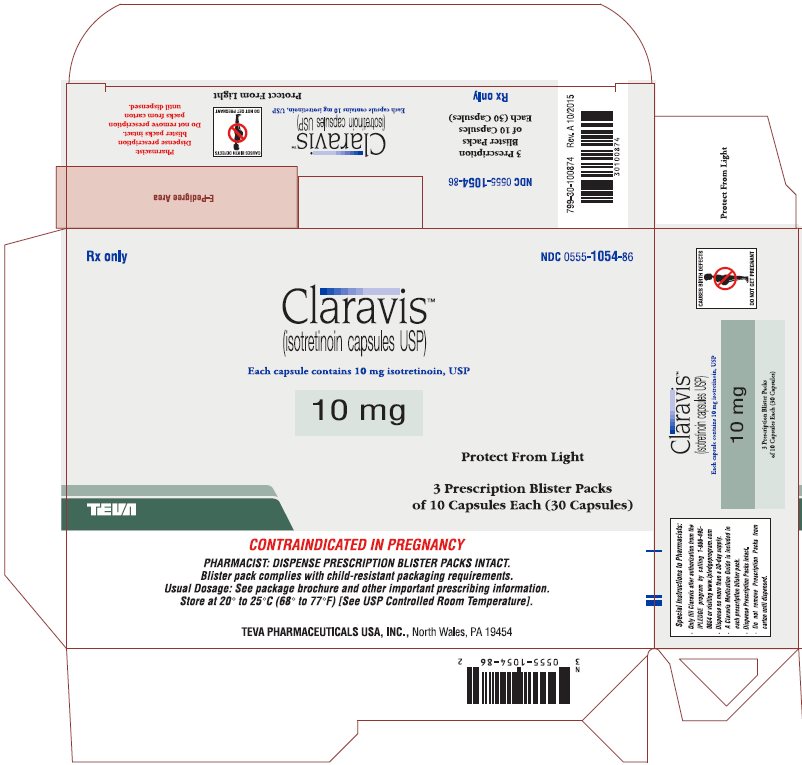
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
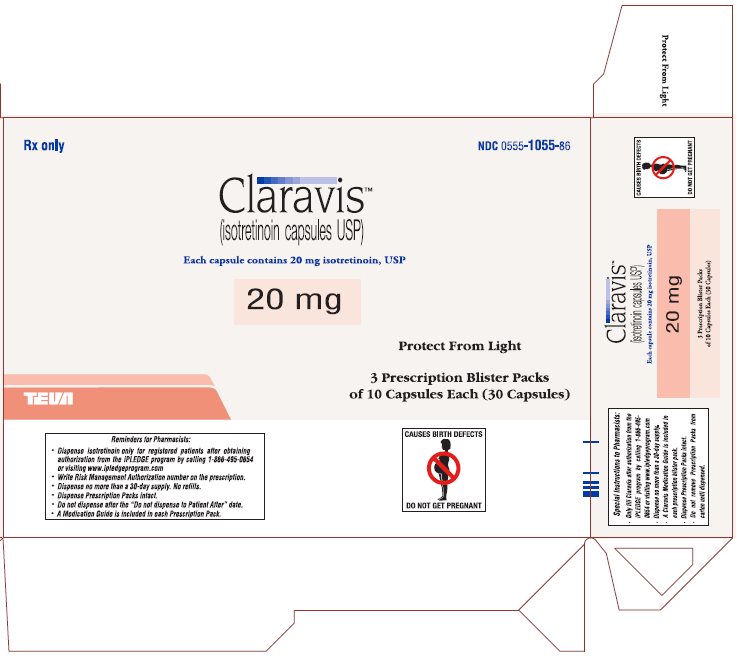
Rx only
NDC 0555-1055-86
ClaravisTM
(isotretinoin capsules USP)
Each capsule contains 20 mg isotretinoin, USP
20 mg
Protect from Light
3 Prescription Blister Packs
of 10 Capsules Each (30 Capsules)
CONTRAINDICATED IN PREGNANCY
PHARMACIST: DISPENSE PRESCRIPTION BLISTER PACKS INTACT.
Blister pack complies with child-resistant packaging requirements.
Usual Dosage: See package brochure and other important prescribing information.
Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature].
TEVA PHARMACEUTICALS USA, Inc., North Wales, PA 19454
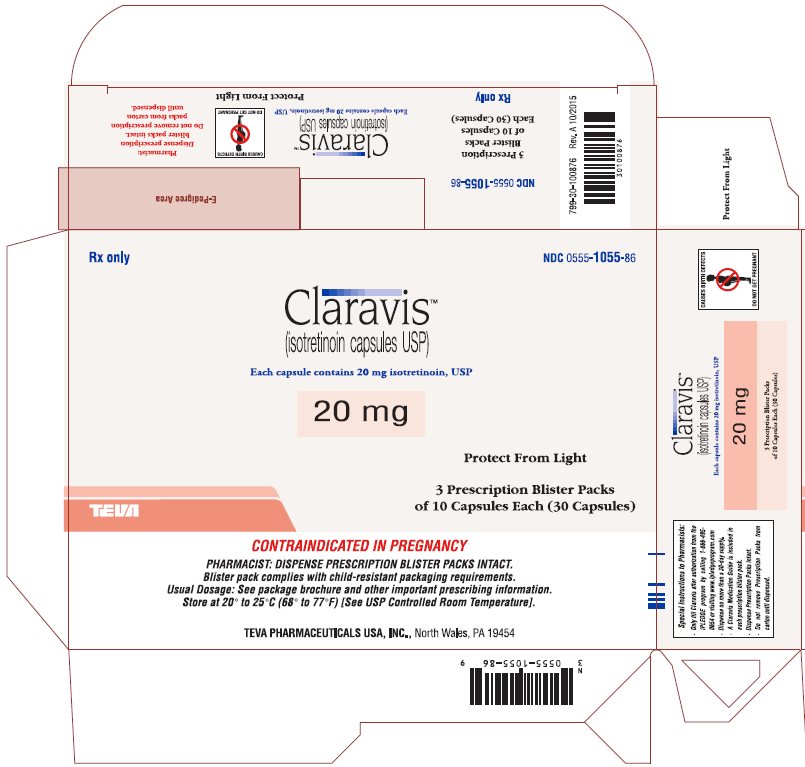
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
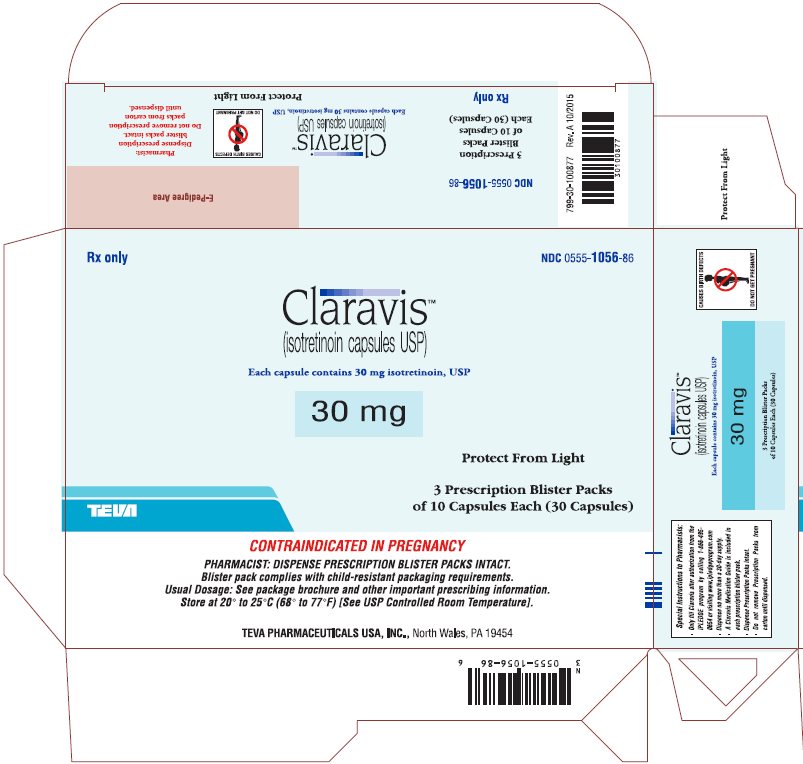
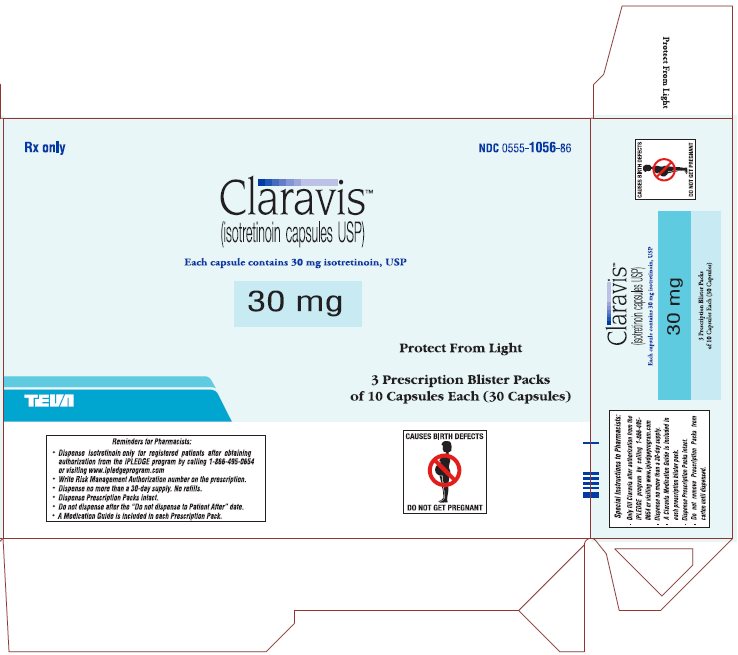
Rx only
NDC 0555-1056-86
ClaravisTM
(isotretinoin capsules USP)
Each capsule contains 30 mg isotretinoin, USP
30 mg
Protect from Light
3 Prescription Blister Packs
of 10 Capsules Each (30 Capsules)
CONTRAINDICATED IN PREGNANCY
PHARMACIST: DISPENSE PRESCRIPTION BLISTER PACKS INTACT.
Blister pack complies with child-resistant packaging requirements.
Usual Dosage: See package brochure and other important prescribing information.
Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature].
TEVA PHARMACEUTICALS USA, Inc., North Wales, PA 19454
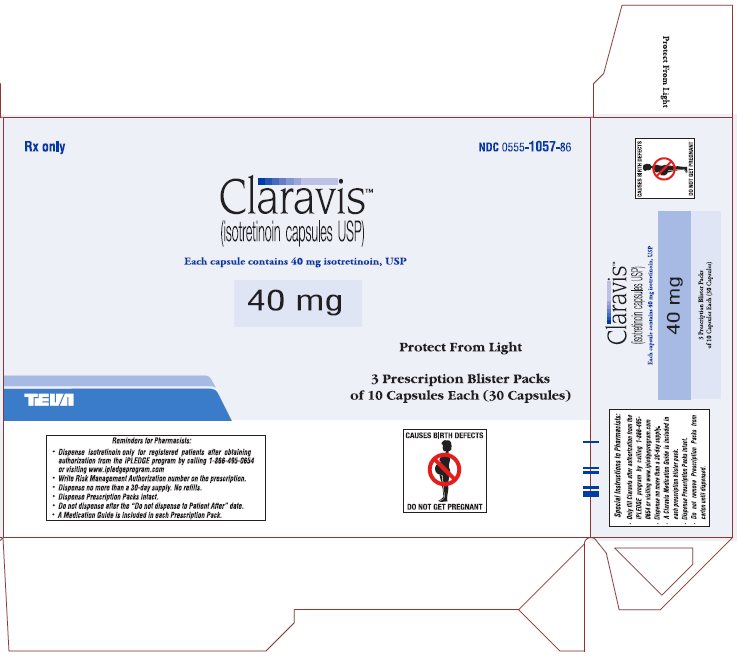
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
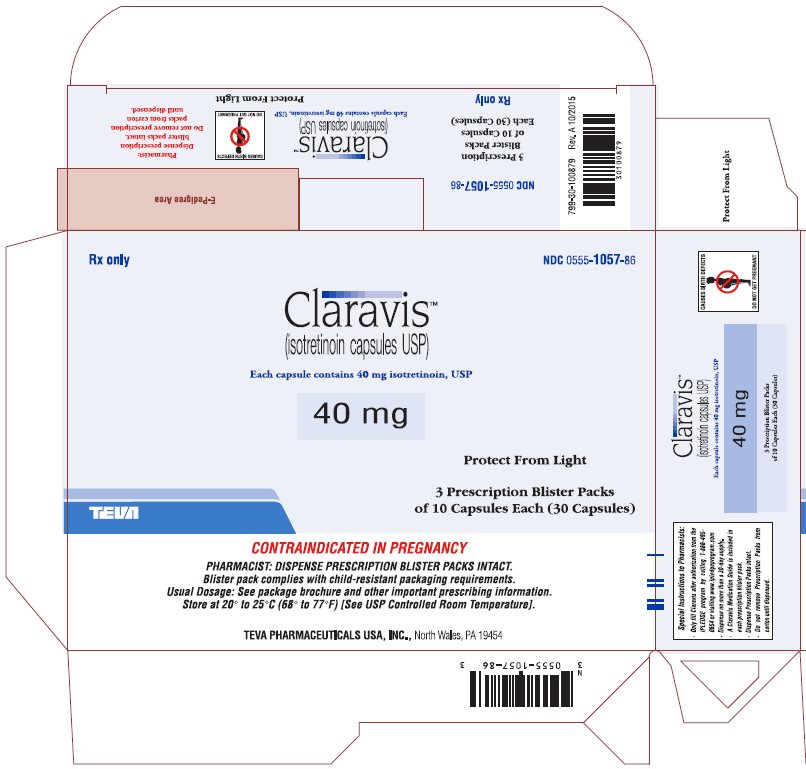
Rx only
NDC 0555-1057-86
ClaravisTM
(isotretinoin capsules USP)
Each capsule contains 40 mg isotretinoin, USP
40 mg
Protect from Light
3 Prescription Blister Packs
of 10 Capsules Each (30 Capsules)
CONTRAINDICATED IN PREGNANCY
PHARMACIST: DISPENSE PRESCRIPTION BLISTER PACKS INTACT.
Blister pack complies with child-resistant packaging requirements.
Usual Dosage: See package brochure and other important prescribing information.
Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature].
TEVA PHARMACEUTICALS USA, Inc., North Wales, PA 19454
PRINCIPAL DISPLAY PANEL