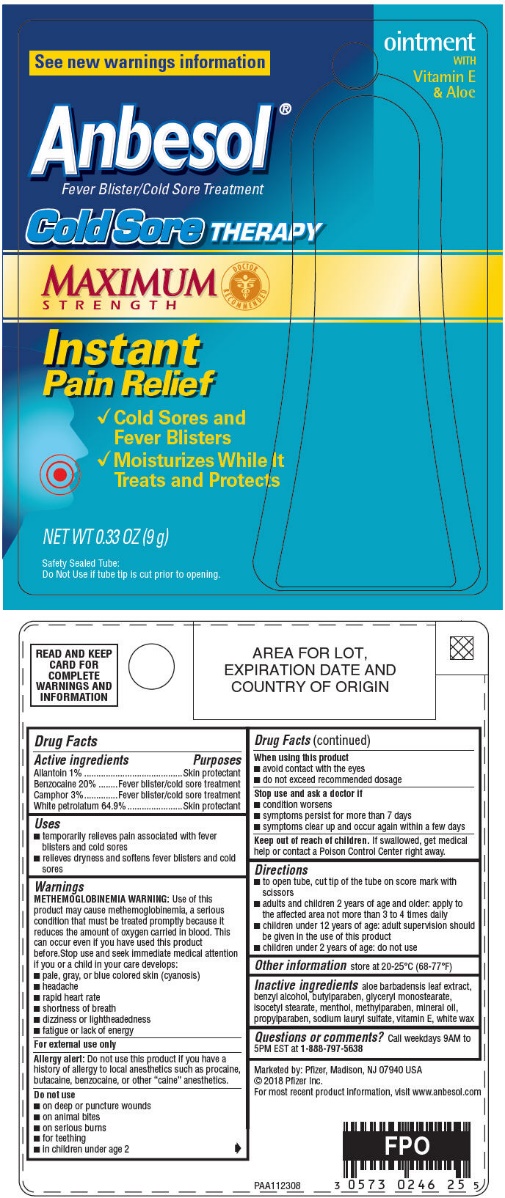
NDC Code(s) : 0573-0246-25, 0573-0246-26
Packager : GlaxoSmithKline Consumer Healthcare Holdings (US) LLC
Category : HUMAN OTC DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| ANBESOL COLD SORE THERAPYallantion, benzocaine, camphor, petrolatum OINTMENT | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| LABELER - GlaxoSmithKline Consumer Healthcare Holdings (US) LLC(079944263) |
PRINCIPAL DISPLAY PANEL
ointment
WITH
Vitamin E
& Aloe
See new warnings information
Anbesol®
Fever Blister/Cold Sore Treatment
Cold Sore THERAPY
MAXIMUM
STRENGTH
DOCTOR
RECOMMENDED
Instant
Pain Relief
-
1.Cold Sores and
Fever Blisters -
2.Moisturizes While It
Treats and Protects
NET WT 0.33 OZ (9 g)
Safety Sealed Tube:
Do Not Use if tube tip is cut prior to opening.