NDC Code(s) : 0591-3449-01, 0591-3449-30, 0591-3450-01, 0591-3450-30, 0591-3451-01, 0591-3451-30, 0591-3452-01, 0591-3452-30, 0591-3576-01, 0591-3576-30, 0591-3453-01, 0591-3453-30
Packager : Watson Laboratories, Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : CII
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Morphine SulfateMorphine Sulfate CAPSULE, EXTENDED RELEASE | ||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| Morphine SulfateMorphine Sulfate CAPSULE, EXTENDED RELEASE | ||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| Morphine SulfateMorphine Sulfate CAPSULE, EXTENDED RELEASE | ||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| Morphine SulfateMorphine Sulfate CAPSULE, EXTENDED RELEASE | ||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
| Morphine SulfateMorphine Sulfate CAPSULE, EXTENDED RELEASE | ||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Morphine SulfateMorphine Sulfate CAPSULE, EXTENDED RELEASE | ||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
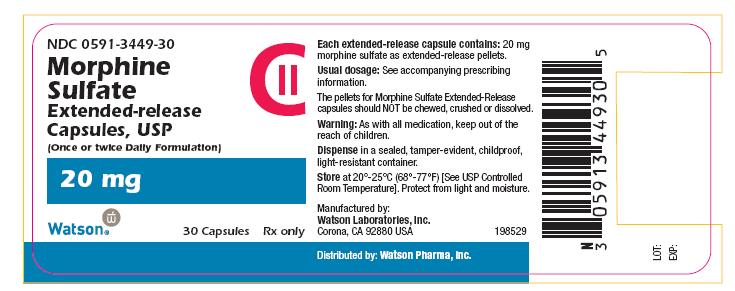
PRINCIPAL DISPLAY PANEL
NDC 0591-3449-30
CII
Morphine
Sulfate
Extended-release
Capsules, USP
(Once or twice Daily Formulation)
20 mg
Watson® 30 Capsules Rx only
PRINCIPAL DISPLAY PANEL
NDC 0591-3450-30
CII
Morphine
Sulfate
Extended-release
Capsules, USP
(Once or twice Daily Formulation)
30 mg
Watson® 30 Capsules Rx only

PRINCIPAL DISPLAY PANEL
NDC 0591-3451-30
CII
Morphine
Sulfate
Extended-release
Capsules, USP
(Once or twice Daily Formulation)
50 mg
Watson® 30 Capsules Rx only

PRINCIPAL DISPLAY PANEL
NDC 0591-3452-30
CII
Morphine
Sulfate
Extended-release
Capsules, USP
(Once or twice Daily Formulation)
60 mg
Watson® 30 Capsules Rx only

PRINCIPAL DISPLAY PANEL
NDC 0591-3576-30
CII
Morphine
Sulfate
Extended-release
Capsules, USP
(Once or twice Daily Formulation)
80 mg
Watson® 30 Capsules Rx only

PRINCIPAL DISPLAY PANEL
NDC 0591-3453-30
CII
Morphine
Sulfate
Extended-release
Capsules, USP
(Once or twice Daily Formulation)
100 mg
Watson® 30 Capsules Rx only










