NDC Code(s) : 0603-5914-20, 0603-5914-16, 0603-5914-32, 0603-5915-20, 0603-5915-16, 0603-5915-32, 0603-5916-20, 0603-5916-16, 0603-5916-32
Packager : Qualitest Pharmaceuticals
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Telmisartan Telmisartan TABLET | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Telmisartan Telmisartan TABLET | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Telmisartan Telmisartan TABLET | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
PRINCIPAL DISPLAY PANEL
Telmisartan Tablets, USP 20 mg (30 Tablets in 1 Bottle)
Each tablet contains 20 mg of telmisartan USP
NDC 0603-5914-16

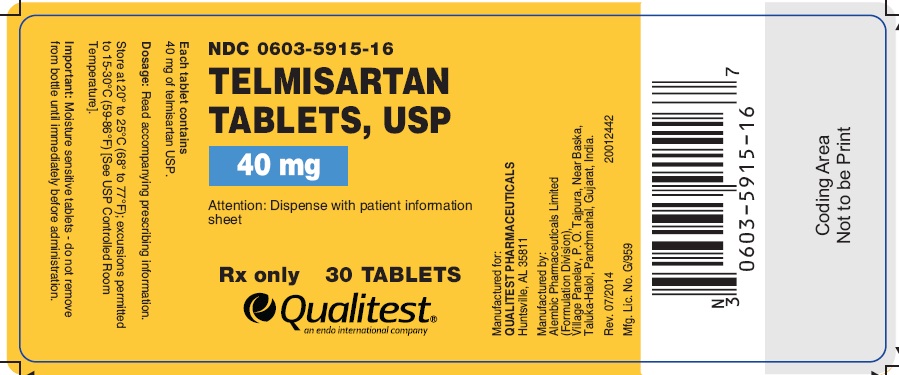
PRINCIPAL DISPLAY PANEL
Telmisartan Tablets, USP 40 mg (30 Tablets in 1 Bottle)
Each tablet contains 40 mg of telmisartan USP
NDC 0603-5915-16
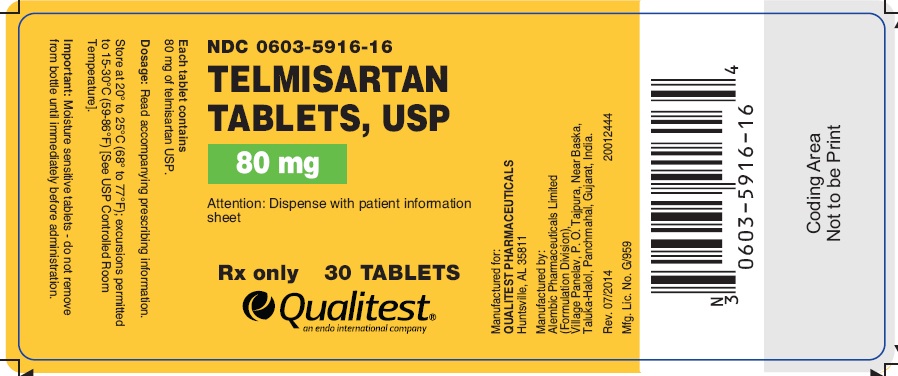
PRINCIPAL DISPLAY PANEL
Telmisartan Tablets, USP 80 mg (30 Tablets in 1 Bottle)
Each tablet contains 80 mg of telmisartan USP
NDC 0603-5916-16