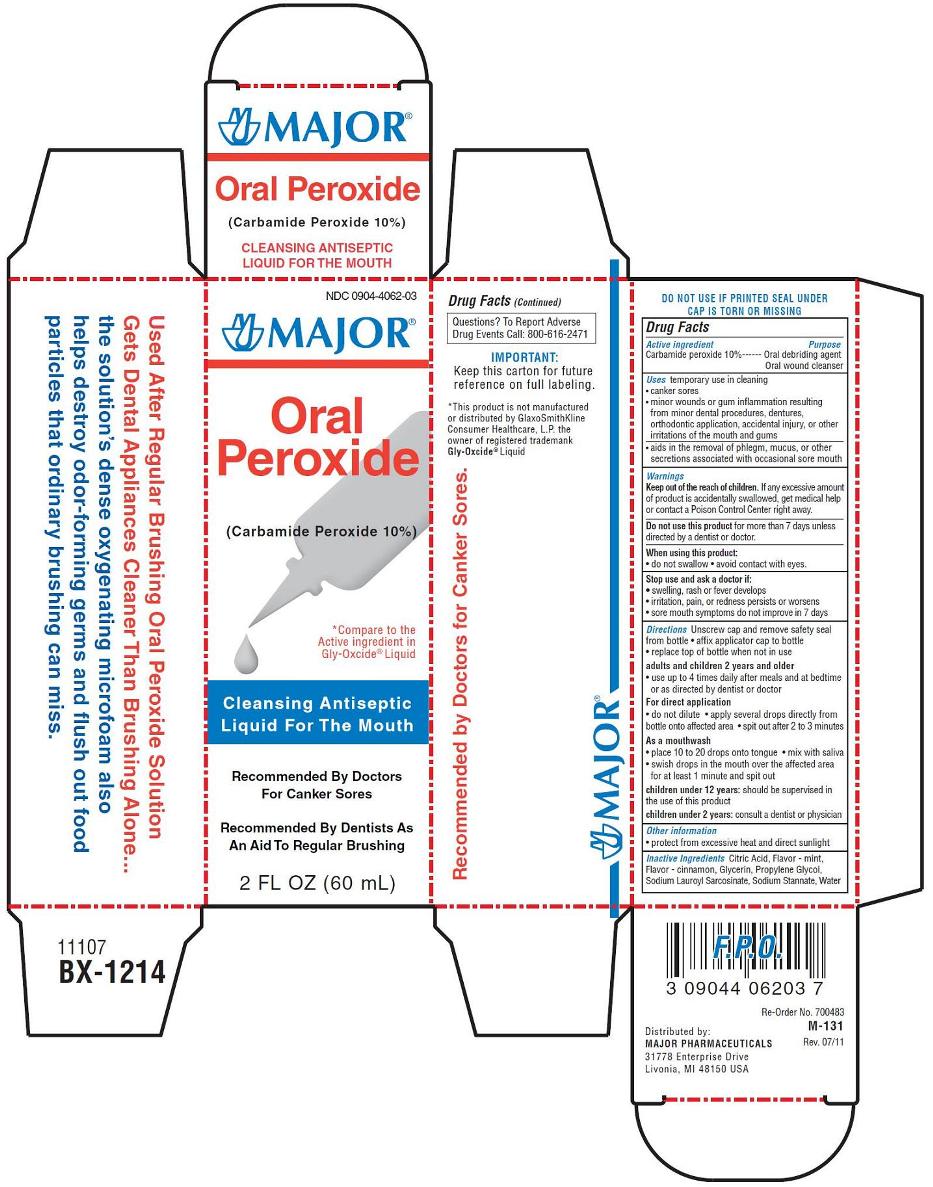
NDC Code(s) : 0904-4062-03
Packager : Major Pharmaceuticals
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Oral Peroxidecarbamide peroxide LIQUID | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
PRINCIPAL DISPLAY PANEL
NDC 0904-4062-03
Oral Peroxide
(Carbamide Peroxide 10%)
ANTISEPTIC ORAL CLEANSER
For minor mouth or gum irritations
Recommended by Doctors and Dentists
- Cleanses dental appliance irritations
- Soothes canker sores
- Kills odor-forming germs
2 fl oz (60 ml)