NDC Code(s) : 0904-5314-35
Packager : Major Pharmaceuticals
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Sodium Chloride Hypertonicitysodium chloride SOLUTION | ||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
PRINCIPAL DISPLAY PANEL
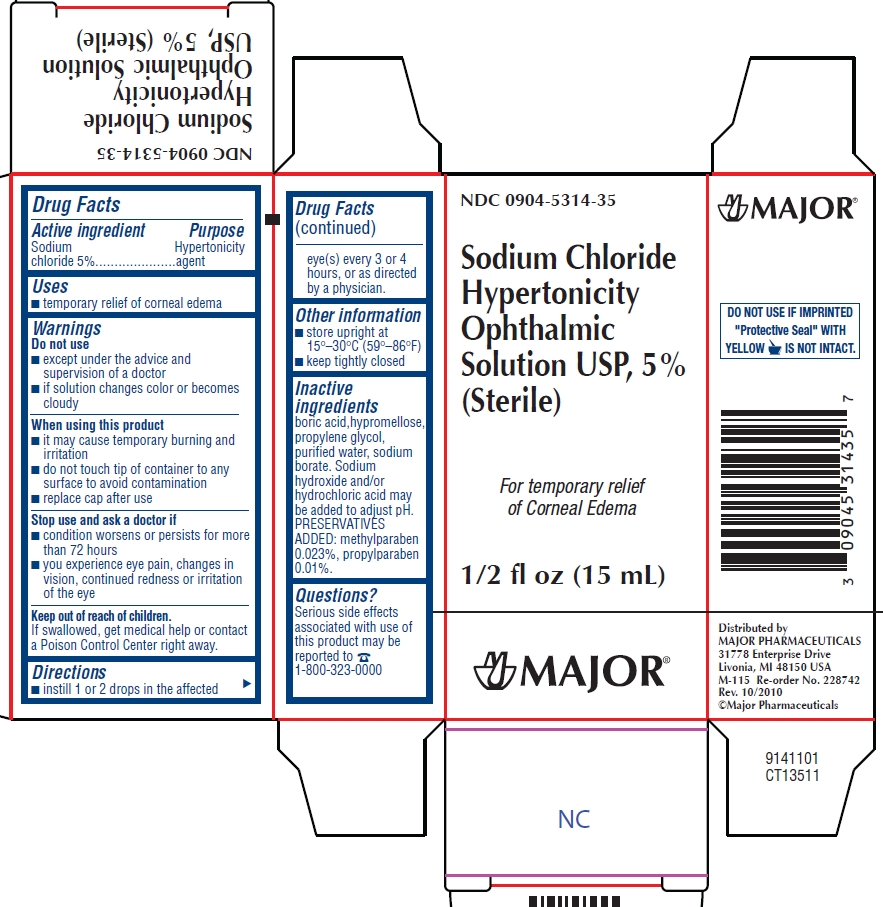
NDC 0904-5314-35
Sodium Chloride Hypertonicity Ophthalmic Solution USP, 5%
(Sterile)
For temporary relief of Corneal Edema
1/2 fl oz (15mL)
MAJOR®









