NDC Code(s) : 0904-6308-20
Packager : Major Pharmaceuticals
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Childrens MAPAPAcetaminophen SUSPENSION | ||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
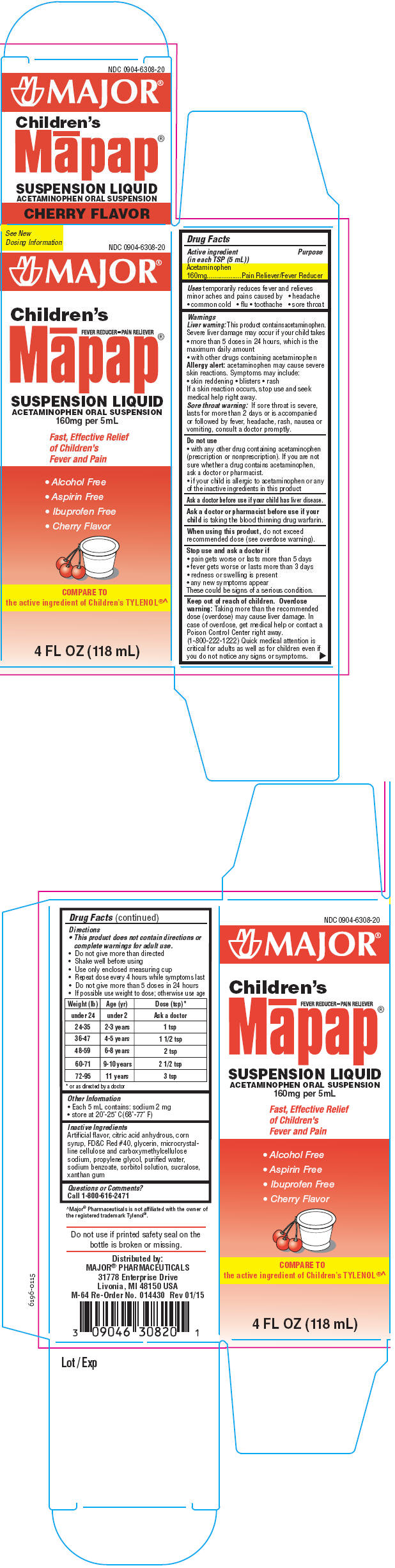
PRINCIPAL DISPLAY PANEL
See New
Dosing Information
NDC 0904-6308-20
MAJOR ®
Children's
FEVER REDUCER-PAIN RELIEVER
Māpap
®
SUSPENSION LIQUID
ACETAMINOPHEN ORAL SUSPENSION
160mg per 5mL
Fast, Effective Relief
of Children's
Fever and Pain
- Alcohol Free
- Aspirin Free
- Ibuprofen Free
- Cherry Flavor
COMPARE TO
the active ingredient of Children's TYLENOL®^
4 FL OZ (118 mL)