NDC Code(s) : 0944-2620-01
Packager : Baxalta US Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| GAMMAGARD Human Immunoglobulin G KIT | |||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
PRINCIPAL DISPLAY PANEL
 GAMMAGARD SD IgA less than or equal to 2.2 ug/mL 2.5g unit carton
GAMMAGARD SD IgA less than or equal to 2.2 ug/mL 2.5g unit carton
GAMMAGARD SD IgA less than or equal to 2.2 ug/mL 2.5g unit carton
50 mL size, dried
NDC 0944-2623-02
Immune Globulin Intravenous (Human)
GAMMAGARD S/D
IgA less than or equal to 2.2 µg/mL in a 5% solution
2.5g
Single dose container. For Intravenous administration only.
Dosage and Administration: See enclosed package insert.
Reconstitution Expiry Information: See Dosage and Administration in enclosed package insert.
Storage temperature: Not to exceed 25°C (77°F). Do not freeze.
This product is prepared from large pools of human plasma. Human blood and its components may transmit infectious agents. The patient and physician should discuss the risks and benefits of this product.
Rx Only
Baxalta US Inc.
Westlake Village, CA 91362 USA
U.S. License No. 2020
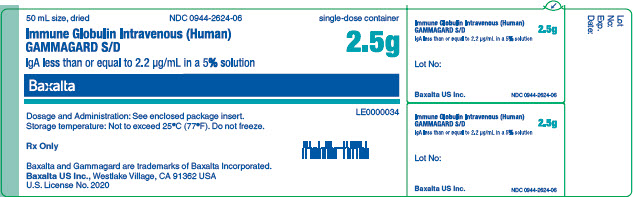 GAMMAGARD SD IgA less than or equal to 2.2 ug/mL 2.5g vial label
GAMMAGARD SD IgA less than or equal to 2.2 ug/mL 2.5g vial label
GAMMAGARD SD IgA less than or equal to 2.2 ug/mL 2.5g vial label
50mL size, dried
NDC 0944-2620-06
single dose container
Immune Globulin Intravenous (Human)
GAMMAGARD S/D
IgA less than or equal to 2.2 µg/mL in a 5% solution
2.5g
Dosage and Administration: See enclosed package insert.
Storage temperature: not to exceed 25°C (77°F). Do not freeze.
Rx Only
Baxalta and Gammagard are trademarks of Baxalta Incorporated.
Baxalta US Inc., Westlake Village, CA 91362 USA
U.S. License No. 2020

GAMMAGARD SD IgA less than or equal to 2.2 ug/mL 5g unit carton
96 mL size dried
NDC 0944-2625-03
Immune Globulin Intravenous (Human)
GAMMAGARD S/D
IgA less than or equal to 2.2 µg/mL in a 5% solution
5g
Single dose container. For Intravenous administration only.
Dosage and Administration: See enclosed package insert.
Reconstitution Expiry Information: See Dosage and Administration in enclosed package insert.
Storage temperature: Not to exceed 25°C (77°F). Do not freeze.
This product is prepared from large pools of human plasma. Human blood and its components may transmit infectious agents. The patient and physician should discuss the risks and benefits of this product.
Rx Only
Baxalta US Inc.
Westlake Village, CA 91362 USA
U.S. License No. 2020
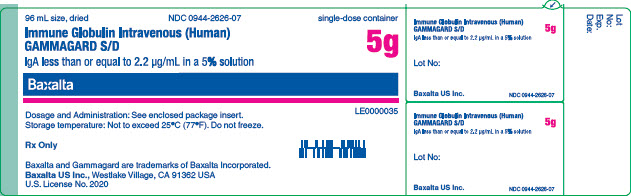
GAMMAGARD SD IgA less than or equal to 2.2 ug/mL 5g vial label
96 mL size, dried
NDC 0944-2620-07
single dose container
Immune Globulin Intravenous (Human)
GAMMAGARD S/D
IgA less than or equal to 2.2 µg/mL in a 5% solution
0.5g
Dosage and Administration: See enclosed package insert.
Storage temperature: not to exceed (25°C (77°F). Do not freeze.
Rx Only
Baxalta and Gammagard are trademarks of Baxalta Incorporated.
Baxalta US Inc., Westlake Village, CA 91362 USA
U.S. License No. 2020

GAMMAGARD SD IgA less than or equal to 2.2 ug/mL 10g unit carton
192mL size, dried
NDC 0944-2627-04
single dose container
Immune Globulin Intravenous (Human)
GAMMAGARD S/D
IgA less than or equal to 2.2 µg/mL in a 5% solution
10g
Single dose container. For Intravenous administration only.
Dosage and Administration: See enclosed package insert.
Reconstitution Expiry Information: See Dosage and Administration in enclosed package insert.
Storage temperature: Not to exceed 25°C (77°F). Do not freeze.
This product is prepared from large pools of human plasma. Human blood and its components may transmit infectious agents. The patient and physician should discuss the risks and benefits of this product.
Rx Only
Baxalta US Inc.
Westlake Village, CA 91362 USA
U.S. License No. 2020

GAMMAGARD SD IgA less than or equal to 2.2 ug/mL 10g vial label
192 mL size, dried
NDC 0944-2628-08
single dose container
Immune Globulin Intravenous (Human)
GAMMAGARD S/D
IgA less than or equal to 2.2 µg/mL in a 5% solution
10g
Dosage and Administration: See enclosed package insert.
Storage temperature: not to exceed 25°C (77°F). Do not freeze.
Rx Only
Baxalta and Gammagard are trademarks of Baxalta Incorporated.
Baxalta US Inc., Westlake Village, CA 91362 USA
U.S. License No. 2020
 Sterile Water for Injection, USP 50 mL
Sterile Water for Injection, USP 50 mL
Sterile Water for Injection, USP 50 mL
Nonpyrogenic
NDC 0338-0001-65
50 ml
Single-Dose Container
Baxter
Sterile Water for Injection, USP
for reconstitution of accompanying product
See accompanying directions for use. Do not use unless clear. No antimicrobial
agent or other substance has been added. Do not use for intravascular injection
without making approximately isotonic by addition of suitable solute. Discard unused portion.
Rx Only.
Manufactured by
Baxter Healthcare Corporation
Deerfield, IL 60015 USA
Imported by
Baxter Corporation
Mississauga, ON L5N 0C2
Baxter is a trademark of Baxter International Inc.

Sterile Water for Injection, USP 96 mL
Nonpyrogenic
NDC 0338-0001-77
96 mL
Single-Dose Container
DIN 02214733
Sterile Water for Injection, USP
for reconstitution of accompanying product
See accompanying directions for use. Do not use unless clear. No
antimicrobial agent or other substance has been added. Do not use for
intravascular injection without making approximately isotonic by addition of
suitable solute. Discard unused portion.
Rx Only.
Manufactured by
Baxter Healthcare Corporation
Deerfield, IL 60015 USA
Imported by
Baxter Corporation
Mississauga, ON L5N 0C2
Baxter is a trademark of
Baxter International Inc.
 Sterile Water for Injection, USP 192 mL
Sterile Water for Injection, USP 192 mL
Nonpyrogenic
NDC 0338-0001-37
192 mL
Single-Dose Container
DIN 02214733
Sterile Water for Injection, USP
for reconstitution of accompanying product
See accompanying directions for use. Do not use unless clear. No
antimicrobial agent or other substance has been added. Do not use for
intravascular injection without making approximately isotonic by addition of
suitable solute. Discard unused portion.
Rx Only.
Manufactured by
Baxter Healthcare Corporation
Deerfield, IL 60015 USA
Imported by
Baxter Corporation
Mississauga, ON L5N 0C2
Baxter is a trademark of
Baxter International Inc.









