NDC Code(s) : 0944-4201-08
Packager : Baxter Healthcare Corporation
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| TISSEELfibrinogen human and thrombin human KIT | |||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
PRINCIPAL DISPLAY PANEL
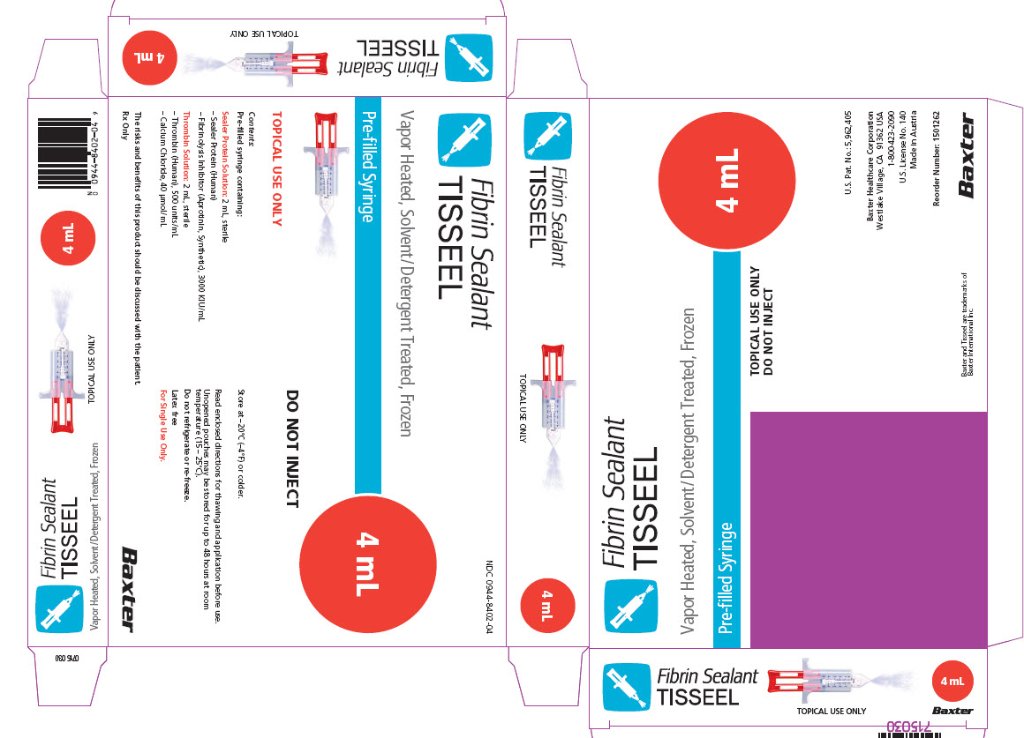 TISSEEL Pre-filled
syringe 4 mL unit carton
TISSEEL Pre-filled
syringe 4 mL unit carton
Fibrin Sealant
TISSEEL
4 mL
NDC 0944-8402-04
Vapor Heated, Solvent/Detergent Treated, Frozen
Pre-filled Syringe
TOPICAL USE ONLY
DO NOT INJECT
Contents:
Pre-filled syringe containing:
Sealer Protein Solution (1): 2 mL, sterile
Sealer Protein (Human)
Fibrinolysis Inhibitor (Aprotinin, Synthetic), 3000 KIU/mL
Thrombin Solution (2): 2 mL, sterile
Thrombin (Human), 500 units/mL
Calcium Chloride, 40 μmol/mL
The risks and benefits of this product should be discussed with the patient.
Rx Only
Store at -20ºC (-4ºF) or colder.
Read directions for thawing and application before use.
Thawed, unopened pouches may be stored for up to 48 hours at room temperature (15-25ºC) after removal from the freezer.
Do not refrigerate or re-freeze.
Latex free
For single use only.
 TISSEEL 4 mL
Pre-filled syringe pouch label
TISSEEL 4 mL
Pre-filled syringe pouch label
Fibrin Sealant
TISSEEL
4 mL
NDC 0944-8402-04
Vapor Heated, Solvent/Detergent Treated, Frozen
Contents:
Pre-filled syringe containing:
Sealer Protein Solution (1): 2 mL, sterile
Sealer Protein (Human)
Fibrinolysis Inhibitor (Aprotinin, Synthetic), 3000 KIU/mL
Thrombin Solution (2): 2 mL, sterile
Thrombin (Human), 500 units/mL
Calcium Chloride, 40 μmol/mL
Read directions for thawing and application before use.
NOT FOR INJECTION.
Store at -20ºC (-4ºF) or colder. Thawed, unopened pouches may be stored for up to 48 hours at room temperature (15-25ºC) after removal from the freezer. Do not refrigerate or re-freeze.
Rx Only
BAXTER and TISSEEL are trademarks of Baxter Healthcare International Inc., registered in the U.S. Patent and Trademark Office.
Baxter Healthcare Corporation
Westlake Village, CA 91362 USA
U.S. License No. 140
 Tisseel 4 mL
Frozen syringe label
Tisseel 4 mL
Frozen syringe label
Fibrin Sealant
TISSEEL 4 mL
 TISSEEL 4 mL kit
unit carton
TISSEEL 4 mL kit
unit carton
Fibrin Sealant
TISSEEL
4 mL
NDC 0944-4201-07
Vapor Heated, Solvent/Detergent Treated, Kit
Contents:
Sealer Protein Concentrate (Human), Vapor Heated, Solvent/Detergent Treated, freeze dried, sterile, for 2 mL of Sealer Protein Solution
Fibrinolysis Inhibitor Solution (Synthetic), sterile, 3000 KIU of Aprotinin/mL
Thrombin (Human), Vapor Heated, Solvent/Detergent Treated, freeze dried, sterile, for 2 mL of Thrombin Solution 500 units/mL
Calcium Chloride Solution, sterile, 40 μmol/mL
The risks and benefits of this product should be discussed with the patient.
Read directions for reconstitution and application before use.
Use within four hours of reconstitution.
NOT FOR INJECTION. For single use only.
Latex free
Rx Only
 Thrombin (Human)
vial label
Thrombin (Human)
vial label
Thrombin (Human)
NDC 0944-7332-02
Vapor Heated, Solvent/Detergent Treated, Freeze-Dried, Sterile
Reconstitute with 2 mL of Calcium Chloride Solution
Concentration: 500 units/mL
Use within 4 hours of reconstitution.
Read enclosed directions prior to use.
NOT FOR INJECTION. Store at 2ºC to 25ºC.
Baxter Healthcare Corporation
Westlake Village, CA 91362 USA
U.S. License No. 140
Rx only
 Calcium Chloride
Solution vial label
Calcium Chloride
Solution vial label
Calcium Chloride Solution
NDC 0944-7401-02
Sterile
For reconstitution of freeze-dried Thrombin (Human),
Contents: 2 mL
Concentration: 40 μmol/mL
NOT FOR INJECTION. Store at 2ºC to 25ºC.
Baxter Healthcare Corporation
Westlake Village, CA 91362 USA
U.S. License No. 140
Rx only
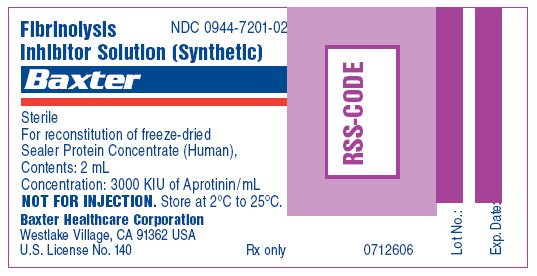 Fibrinolysis
Inhibitor Solution (Synthetic) vial label
Fibrinolysis
Inhibitor Solution (Synthetic) vial label
Fibrinolysis Inhibitor Solution (Synthetic)
NDC 0944-7201-02
Sterile
For reconstitution of freeze-dried Sealer Protein Concentrate (Human),
Contents: 2 mL
Concentration: 3000 KIU of Aprotinin/mL
NOT FOR INJECTION. Store at 2ºC to 25ºC.
Baxter Healthcare Corporation
Westlake Village, CA 91362 USA
U.S. License No. 140
Rx only
 Sealer Protein
Concentrate (Human) vial label
Sealer Protein
Concentrate (Human) vial label
Sealer Protein Concentrate (Human)
NDC 0944-7112-02
Vapor Heated, Solvent/Detergent Treated, Freeze-Dried, Sterile
Reconstitute with 2 mL of Fibrinolysis Inhibitor Solution (Synthetic)
Vial contains a stirrer to facilitate reconstitution.
Use within 4 hours of reconstitution.
Read enclosed directions prior to use.
NOT FOR INJECTION. Store at 2ºC to 25ºC.
Baxter Healthcare Corporation
Westlake Village, CA 91362 USA
U.S. License No. 140
Rx only
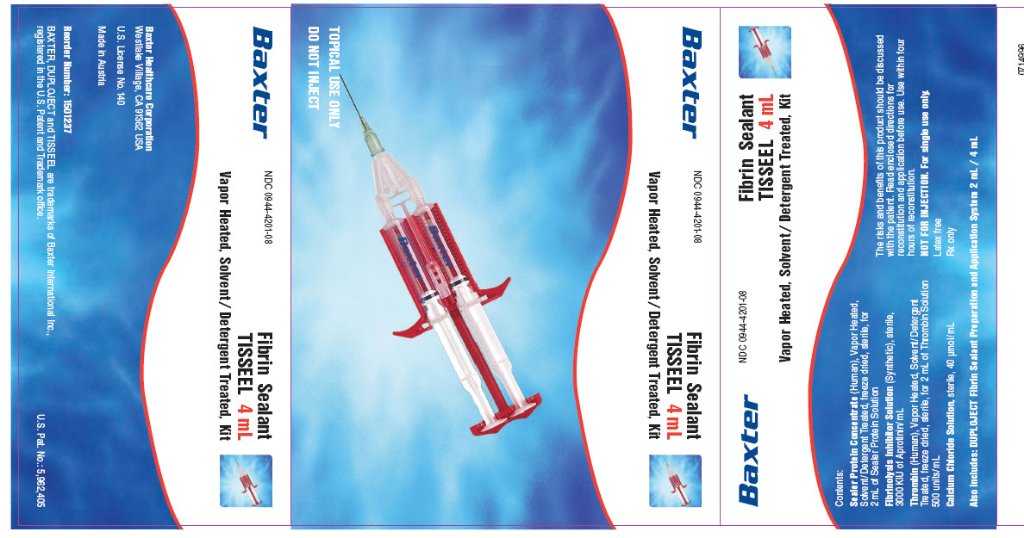 TISSEEL 4 mL kit
with Duploject sleeve
TISSEEL 4 mL kit
with Duploject sleeve
Fibrin Sealant
TISSEEL
4 mL
NDC 0944-4201-08
Vapor Heated, Solvent/Detergent Treated, Kit
Contents:
Sealer Protein Concentrate (Human), Vapor Heated, Solvent/Detergent Treated, freeze dried, sterile, for 2 mL of Sealer Protein Solution
Fibrinolysis Inhibitor Solution (Synthetic), sterile, 3000 KIU of Aprotinin/mL
Thrombin (Human), Vapor Heated, Solvent/Detergent Treated, freeze dried, sterile, for 2 mL of Thrombin Solution 500 units/mL
Calcium Chloride Solution, sterile, 40 μmol/mL
The risks and benefits of this product should be discussed with the patient.
Read directions for reconstitution and application before use.
Use within four hours of reconstitution.
NOT FOR INJECTION. For single use only.
Latex free
Rx Only
Also includes: DUPOLJECT Fibrin Sealant Preparation and Application System 2 mL/ 4 mL
 Duploject 2 mL- 4
mL unit carton
Duploject 2 mL- 4
mL unit carton
DUPLOJECT
2 mL / 4 mL
Fibrin Sealant Preparation and Application System
BAXTER, DUPLOJECT and TISSEEL are trademarks of Baxter International Inc., registered in the U.S. Patent and Trademark office.
Reorder Number: 3400233
Manufactured for:
Baxter Healthcare Corporation
Westlake Village, CA 91362 USA









