NDC Code(s) : 0944-4622-03, 0944-4622-02, 0944-4622-01, 0944-4623-03, 0944-4623-02, 0944-4623-01, 0944-4626-03, 0944-4626-02, 0944-4626-01, 0944-4624-03, 0944-4624-02, 0944-4624-01, 0944-4627-03, 0944-4627-02, 0944-4627-01, 0944-4625-03, 0944-4625-02, 0944-4625-01, 0944-4628-03, 0944-4628-02, 0944-4628-01
Packager : Takeda Pharmaceuticals Amercia, Inc.
Category : PLASMA DERIVATIVE
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| ADYNOVATEAntihemophilic Factor (Recombinant) PEGylated KIT | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| ADYNOVATEAntihemophilic Factor (Recombinant) PEGylated KIT | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| ADYNOVATEAntihemophilic Factor (Recombinant) PEGylated KIT | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| ADYNOVATEAntihemophilic Factor (Recombinant) PEGylated KIT | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| ADYNOVATEAntihemophilic Factor (Recombinant) PEGylated KIT | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| ADYNOVATEAntihemophilic Factor (Recombinant) PEGylated KIT | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| ADYNOVATEAntihemophilic Factor (Recombinant) PEGylated KIT | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| LABELER - Takeda Pharmaceuticals Amercia, Inc.(039997266) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| BAXALTA US INC. | 009471603 | MANUFACTURE(0944-4622, 0944-4623, 0944-4626, 0944-4624, 0944-4627, 0944-4625, 0944-4628) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Siegfried Hameln GmbH | 315869123 | MANUFACTURE(64764-514, 64764-515) | |
PRINCIPAL DISPLAY PANEL
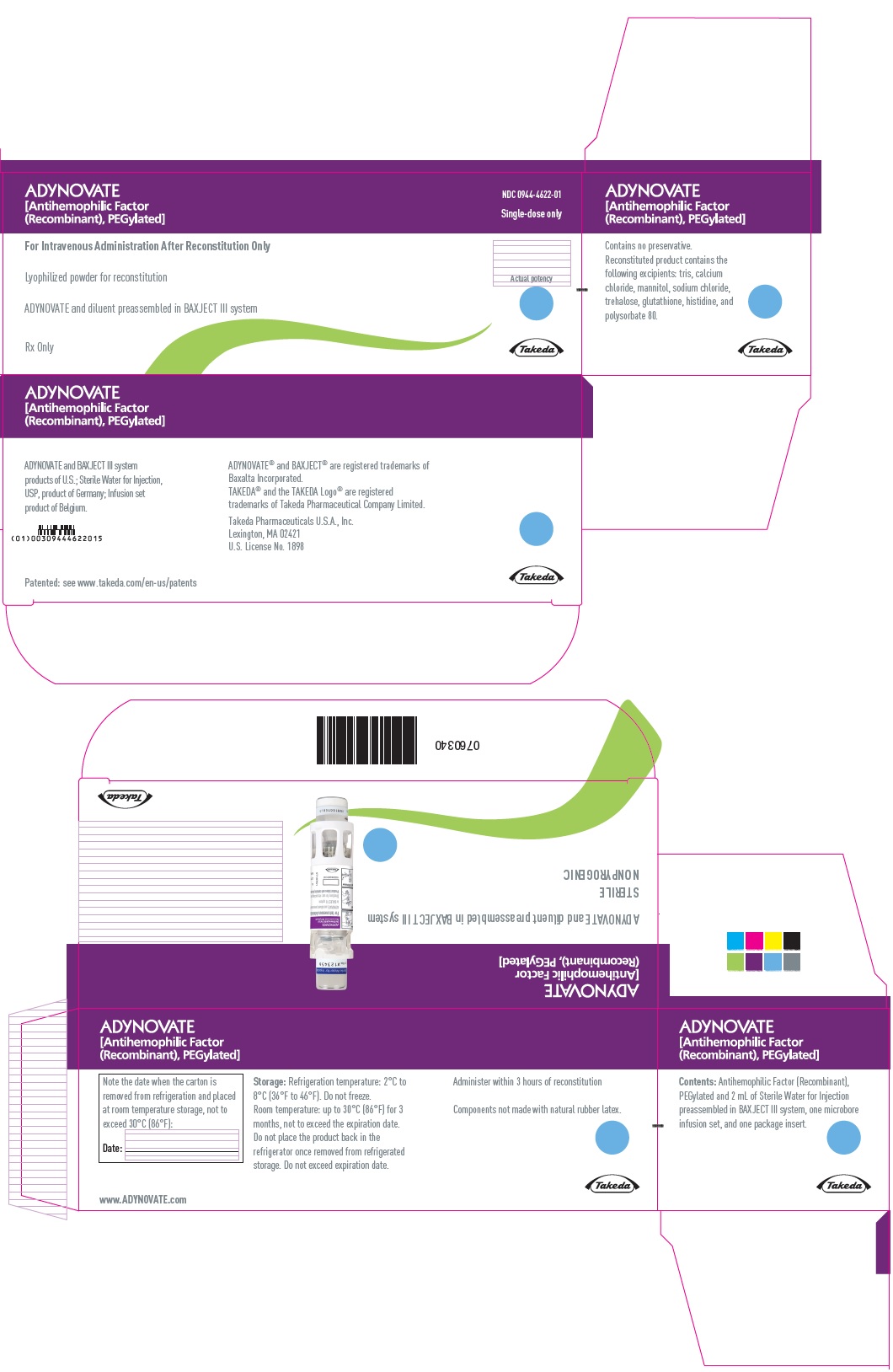
ADYNOVATE
[Antihemophilic Factor
(Recombinant), PEGylated]
NDC 0944-4622-01
Single-dose only
For Intravenous Administration After Reconstitution Only
Lyophilized powder for reconstitution
ADYNOVATE and diluent preassembled in BAXJECT III system
Rx Only
Actual potency
Takeda
PRINCIPAL DISPLAY PANEL
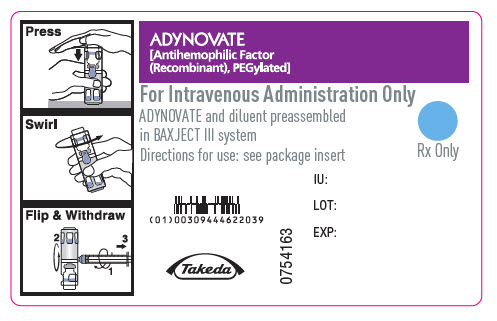
ADYNOVATE
[Antihemophilic Factor
(Recombinant), PEGylated]
For Intravenous Administration Only
ADYNOVATE and diluent preassembled
in BAXJECT III system
Directions for use: see package insert
Rx Only
IU:
LOT:
EXP:
Takeda
0754163
PRINCIPAL DISPLAY PANEL
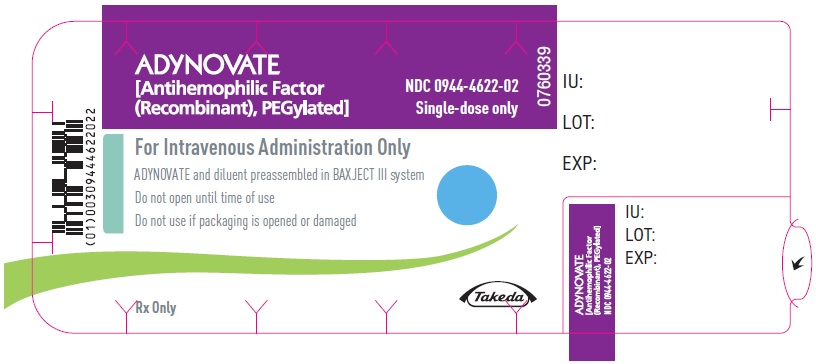
ADYNOVATE
[Antihemophilic Factor
(Recombinant), PEGylated]
NDC 0944-4622-02
Single-dose only
0760339
For Intravenous Administration Only
ADYNOVATE and diluent preassembled in BAXJECT III system
Do not open until time of use
Do not use if packaging is opened or damaged
Rx Only
Takeda
PRINCIPAL DISPLAY PANEL
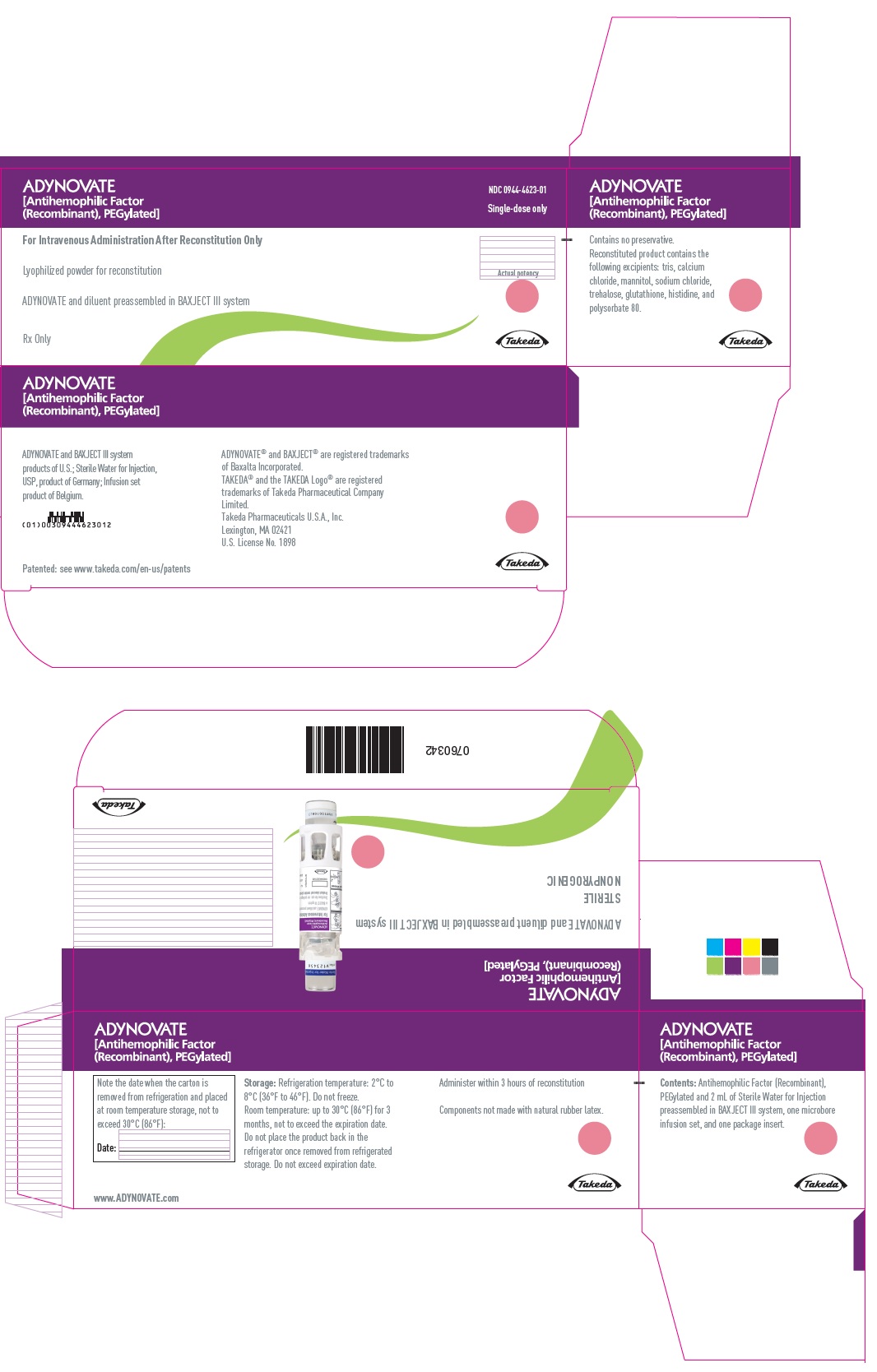
ADYNOVATE
[Antihemophilic Factor
(Recombinant), PEGylated]
NDC 0944-4623-01
Single-dose only
For Intravenous Administration After Reconstitution Only
Lyophilized powder for reconstitution
ADYNOVATE and diluent preassembled in BAXJECT III system
Rx Only
Actual potency
Takeda
PRINCIPAL DISPLAY PANEL
ADYNOVATE
[Antihemophilic Factor
(Recombinant), PEGylated]
For Intravenous Administration Only
ADYNOVATE and diluent preassembled
in BAXJECT III system
Directions for use: see package insert
Rx Only
IU:
LOT:
EXP:
Takeda
0754167

PRINCIPAL DISPLAY PANEL
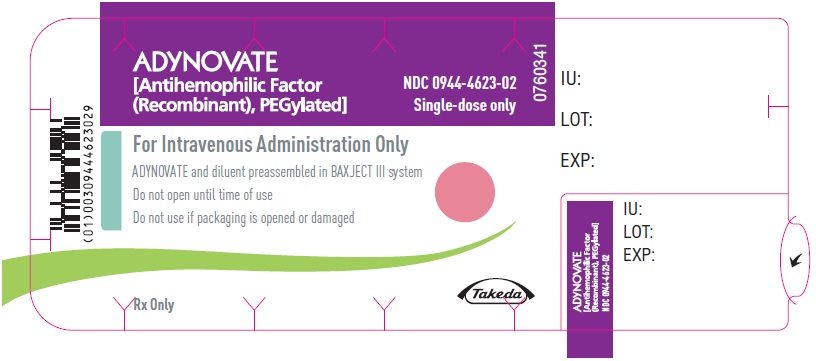
ADYNOVATE
[Antihemophilic Factor
(Recombinant), PEGylated]
NDC 0944-4623-02
Single-dose only
0760341
For Intravenous Administration Only
ADYNOVATE and diluent preassembled in BAXJECT III system
Do not open until time of use
Do not use if packaging is opened or damaged
Rx Only
Takeda
PRINCIPAL DISPLAY PANEL
ADYNOVATE
[Antihemophilic Factor
(Recombinant), PEGylated]
NDC 0944-4626-01
Single-dose only
For Intravenous Administration After Reconstitution Only
Lyophilized powder for reconstitution
ADYNOVATE and diluent preassembled in BAXJECT III system
Rx Only
Actual potency
Takeda
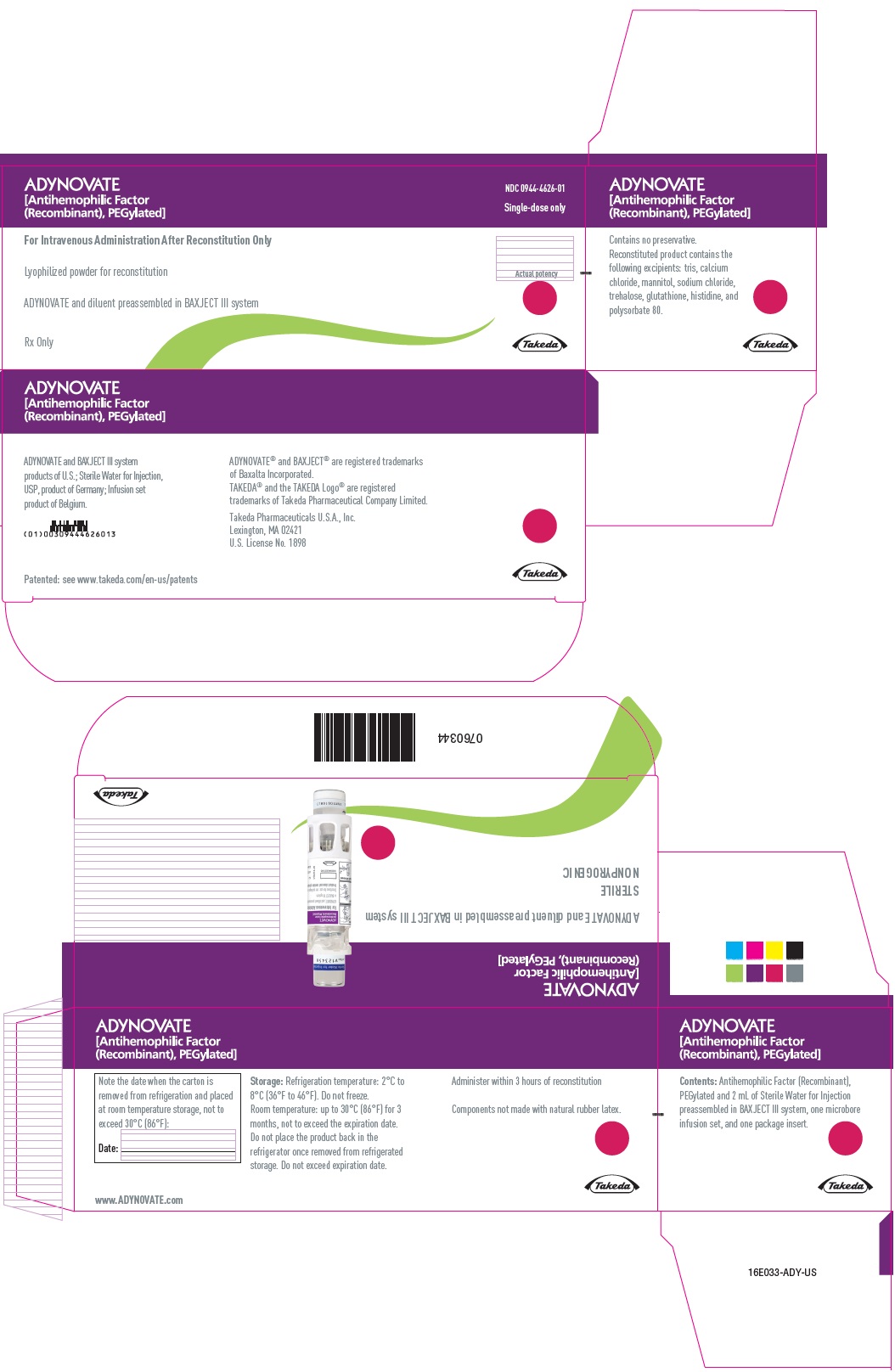
PRINCIPAL DISPLAY PANEL
ADYNOVATE
[Antihemophilic Factor
(Recombinant), PEGylated]
For Intravenous Administration Only
ADYNOVATE and diluent preassembled
in BAXJECT III system
Directions for use: see package insert
Rx Only
IU:
LOT:
EXP:
Takeda
0754182

PRINCIPAL DISPLAY PANEL
ADYNOVATE
[Antihemophilic Factor
(Recombinant), PEGylated]
NDC 0944-4626-02
Single-dose only
0760343
For Intravenous Administration Only
ADYNOVATE and diluent preassembled in BAXJECT III system
Do not open until time of use
Do not use if packaging is opened or damaged
Rx Only
Takeda
PRINCIPAL DISPLAY PANEL
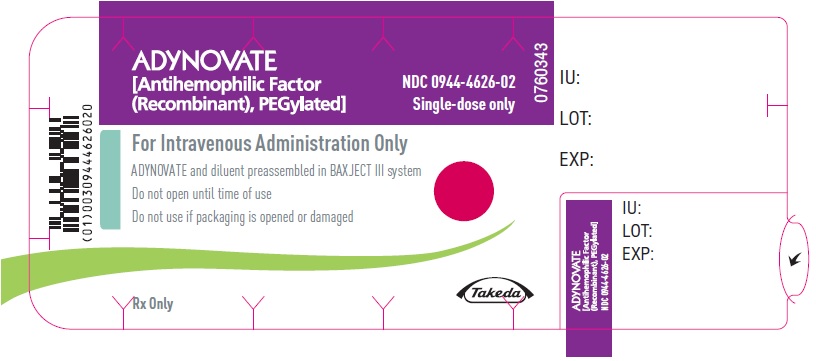
ADYNOVATE
[Antihemophilic Factor
(Recombinant), PEGylated]
NDC 0944-4624-01
Single-dose only
For Intravenous Administration After Reconstitution Only
Lyophilized powder for reconstitution
ADYNOVATE and diluent preassembled in BAXJECT III system
Rx Only
Actual potency
Takeda
PRINCIPAL DISPLAY PANEL
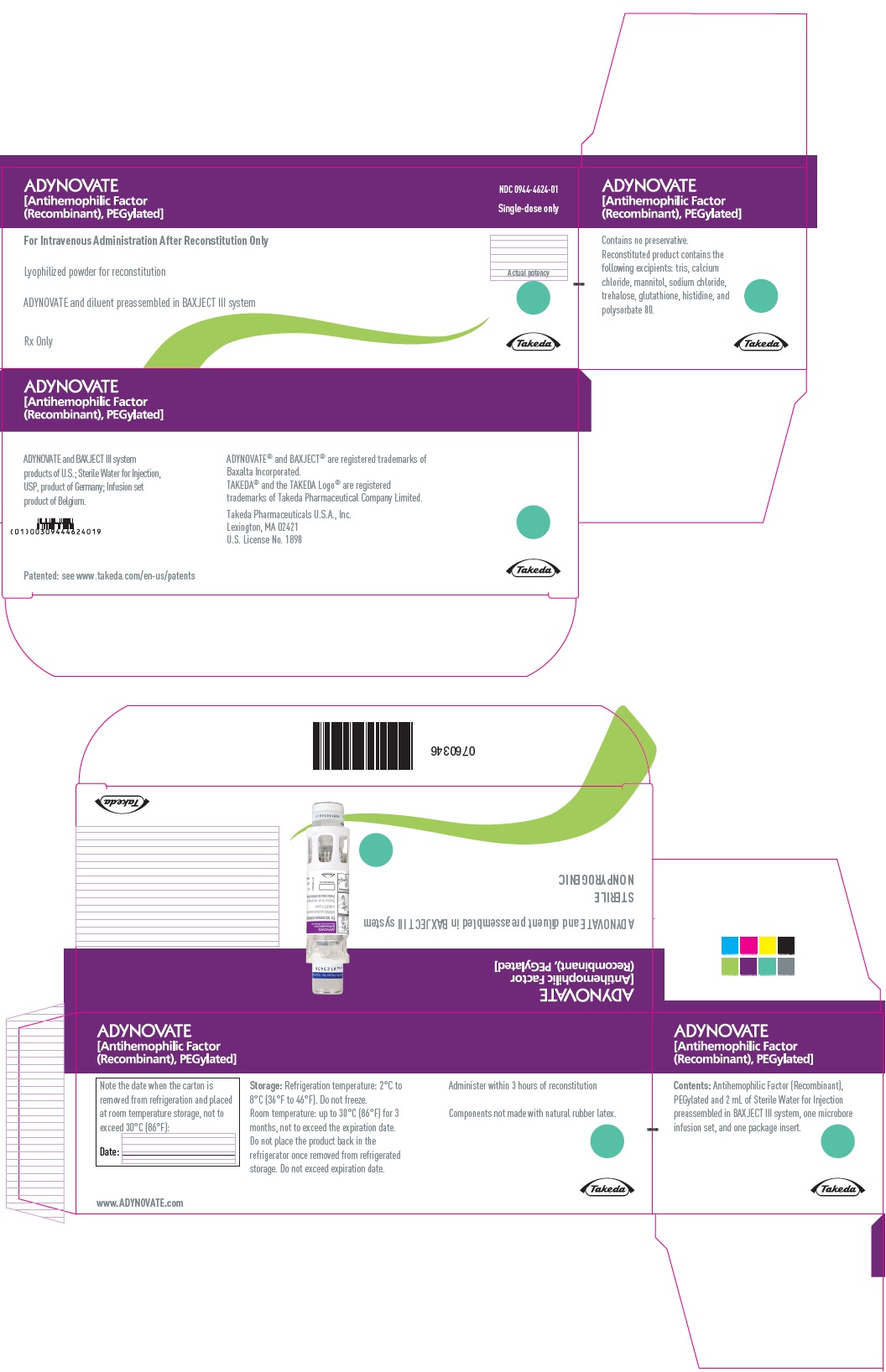
ADYNOVATE
[Antihemophilic Factor
(Recombinant), PEGylated]
For Intravenous Administration Only
ADYNOVATE and diluent preassembled
in BAXJECT III system
Directions for use: see package insert
Rx Only
IU:
LOT:
EXP:
Takeda
0754170

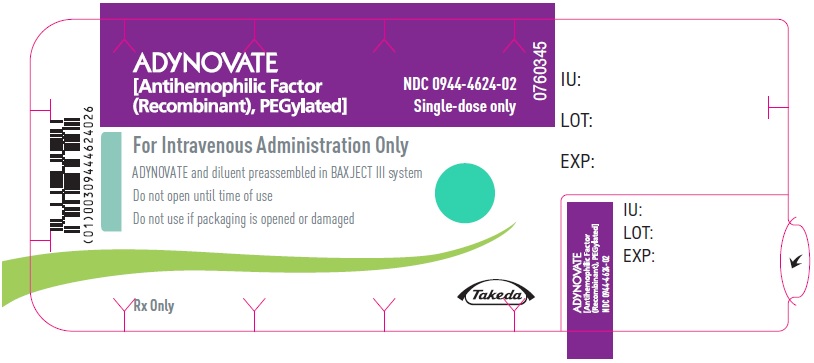
PRINCIPAL DISPLAY PANEL
ADYNOVATE
[Antihemophilic Factor
(Recombinant), PEGylated]
NDC 0944-4624-02
Single-dose only
0760345
For Intravenous Administration Only
ADYNOVATE and diluent preassembled in BAXJECT III system
Do not open until time of use
Do not use if packaging is opened or damaged
Rx Only
Takeda
PRINCIPAL DISPLAY PANEL
ADYNOVATE
[Antihemophilic Factor
(Recombinant), PEGylated]
NDC 0944-4627-01
Single-dose only
For Intravenous Administration After Reconstitution Only
Lyophilized powder for reconstitution
ADYNOVATE and diluent preassembled in BAXJECT III system
Rx Only
Actual potency
Takeda
PRINCIPAL DISPLAY PANEL
ADYNOVATE
[Antihemophilic Factor
(Recombinant), PEGylated]
For Intravenous Administration Only
ADYNOVATE and diluent preassembled
in BAXJECT III system
Directions for use: see package insert
Rx Only
IU:
LOT:
EXP:
Takeda
0754173

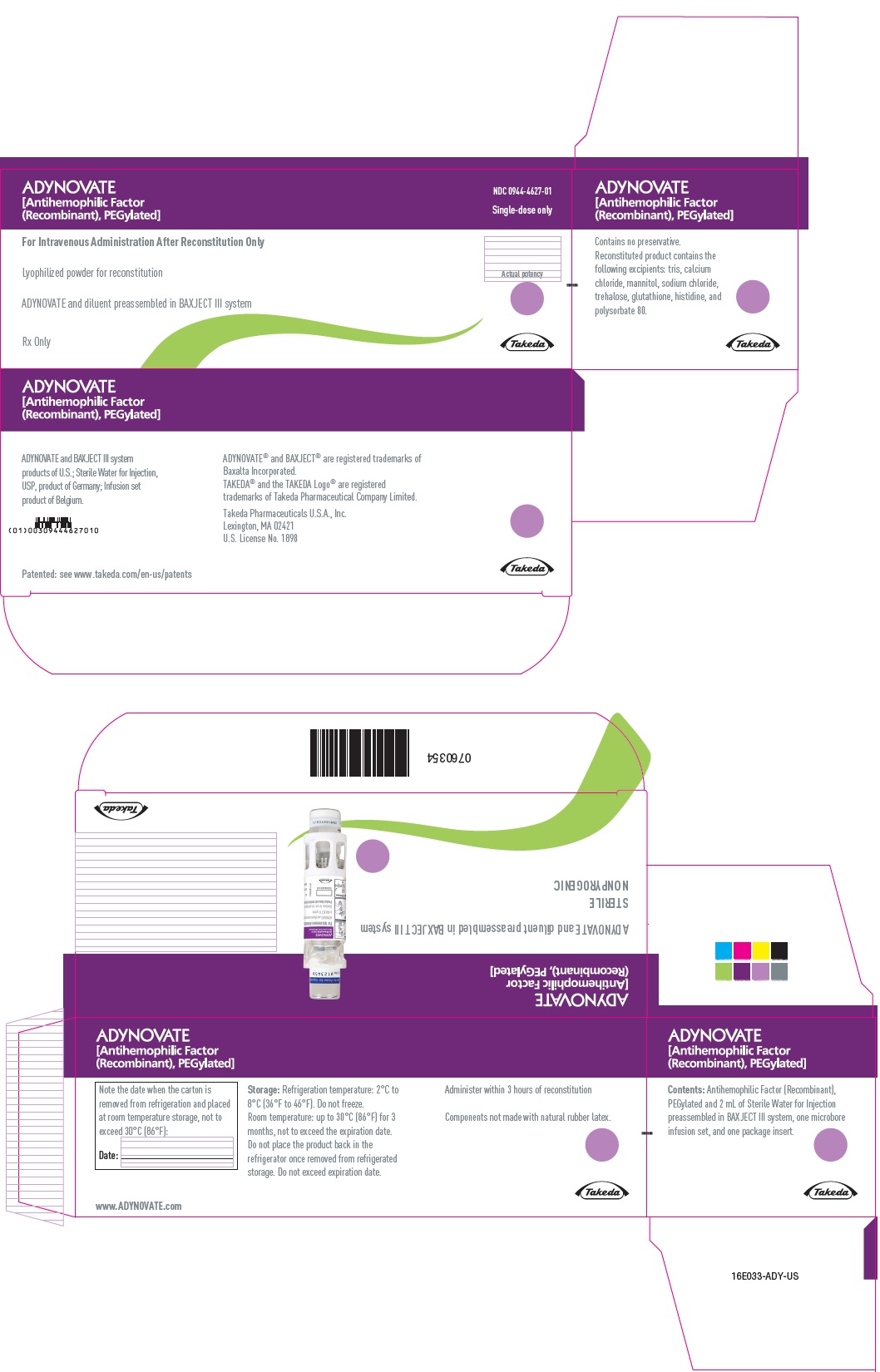
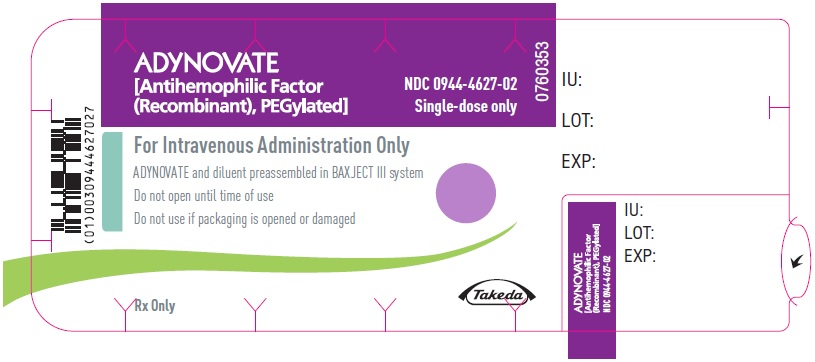
PRINCIPAL DISPLAY PANEL
ADYNOVATE
[Antihemophilic Factor
(Recombinant), PEGylated]
NDC 0944-4627-02
Single-dose only
0760353
For Intravenous Administration Only
ADYNOVATE and diluent preassembled in BAXJECT III system
Do not open until time of use
Do not use if packaging is opened or damaged
Rx Only
Takeda
PRINCIPAL DISPLAY PANEL
ADYNOVATE
[Antihemophilic Factor
(Recombinant), PEGylated]
NDC 0944-4625-01
Single-dose only
Actual potency
For Intravenous Administration After Reconstitution Only
Lyophilized powder for reconstitution
ADYNOVATE and diluent preassembled in
BAXJECT III system
Rx Only
Takeda
PRINCIPAL DISPLAY PANEL
ADYNOVATE
[Antihemophilic Factor
(Recombinant), PEGylated]
For Intravenous Administration Only
ADYNOVATE and diluent preassembled
in BAXJECT III system
Directions for use: see package insert
Rx Only
IU:
LOT:
EXP:
Takeda
0754176
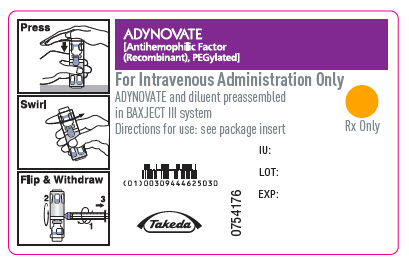
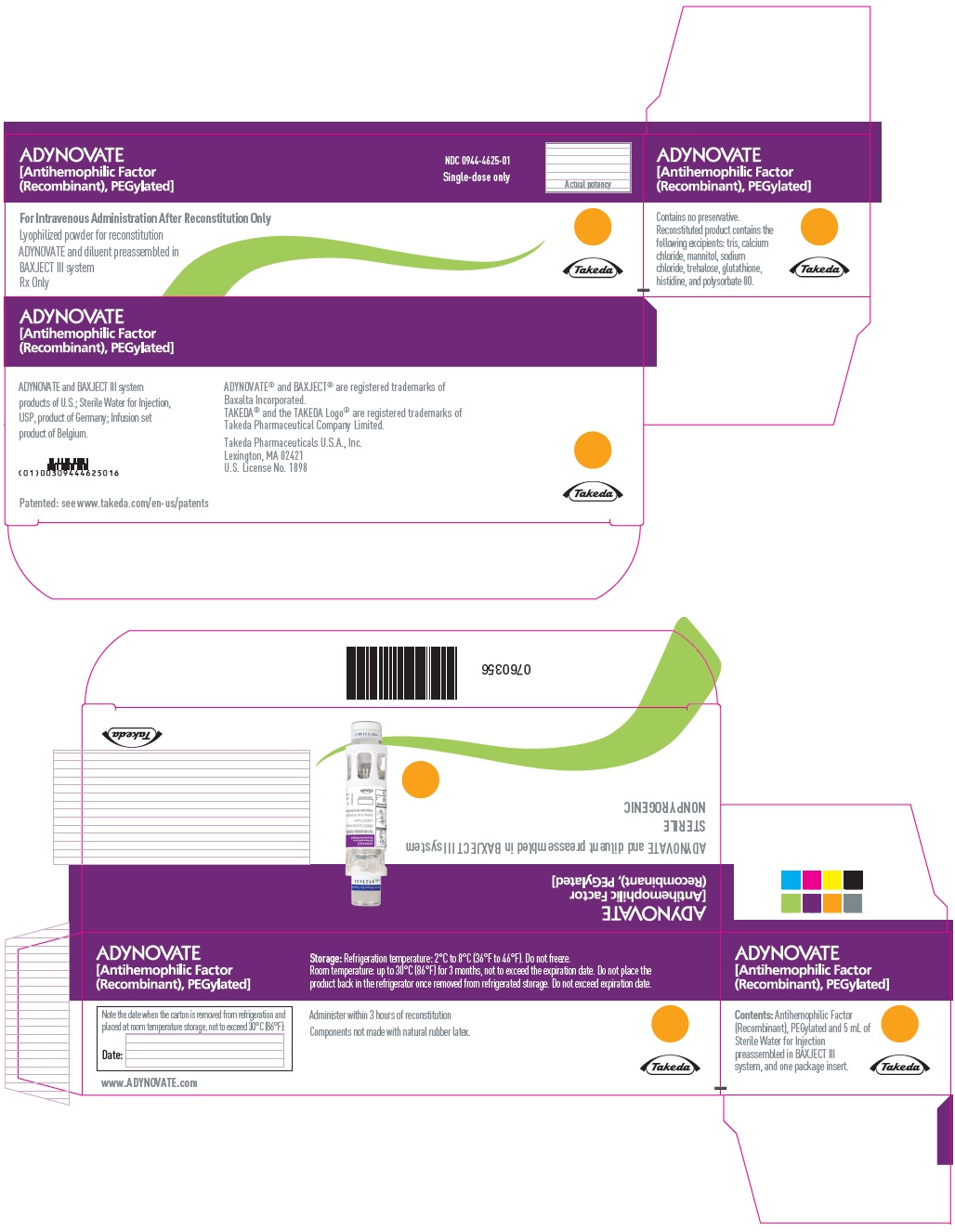
PRINCIPAL DISPLAY PANEL
ADYNOVATE
[Antihemophilic Factor
(Recombinant), PEGylated]
NDC 0944-4625-02
Single-dose only
0760355
For Intravenous Administration Only
ADYNOVATE and diluent preassembled in BAXJECT III system
Do not open until time of use
Do not use if packaging is opened or damaged
Rx Only
Takeda
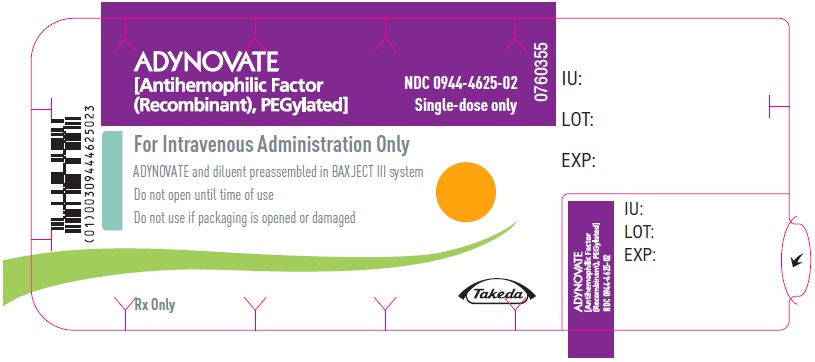
PRINCIPAL DISPLAY PANEL
ADYNOVATE
[Antihemophilic Factor
(Recombinant), PEGylated]
NDC 0944-4628-01
Single-dose only
Actual potency
For Intravenous Administration After Reconstitution Only
Lyophilized powder for reconstitution
ADYNOVATE and diluent preassembled in
BAXJECT III system
Rx Only
Takeda
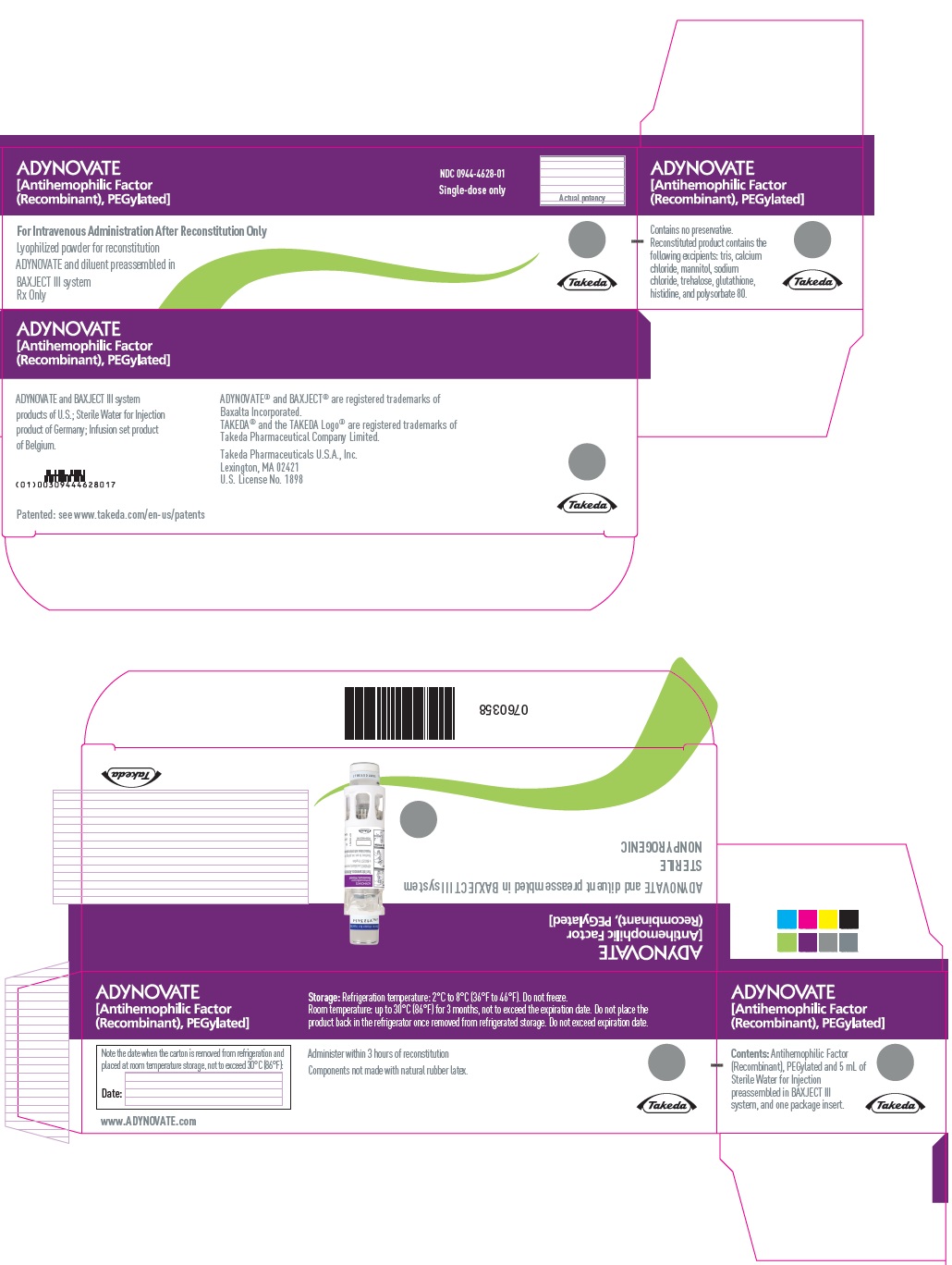
PRINCIPAL DISPLAY PANEL
ADYNOVATE
[Antihemophilic Factor
(Recombinant), PEGylated]
For Intravenous Administration Only
ADYNOVATE and diluent preassembled
in BAXJECT III system
Directions for use: see package insert
Rx Only
IU:
LOT:
EXP:
Takeda
0754179

PRINCIPAL DISPLAY PANEL
ADYNOVATE
[Antihemophilic Factor
(Recombinant), PEGylated]
NDC 0944-4628-02
Single-dose only
0760357
For Intravenous Administration Only
ADYNOVATE and diluent preassembled in BAXJECT III system
Do not open until time of use
Do not use if packaging is opened or damaged
Rx Only
Takeda
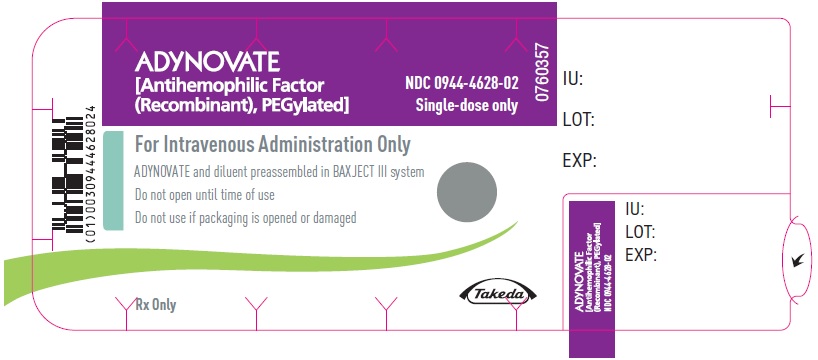
PRINCIPAL DISPLAY PANEL
Antihemophilic Factor (Recombinant), PEGylated
0754164
Takeda
Lot No.:

PRINCIPAL DISPLAY PANEL
Sterile Water for Injection
Lot No.:
Siegfried Hameln GmbH
0742211










