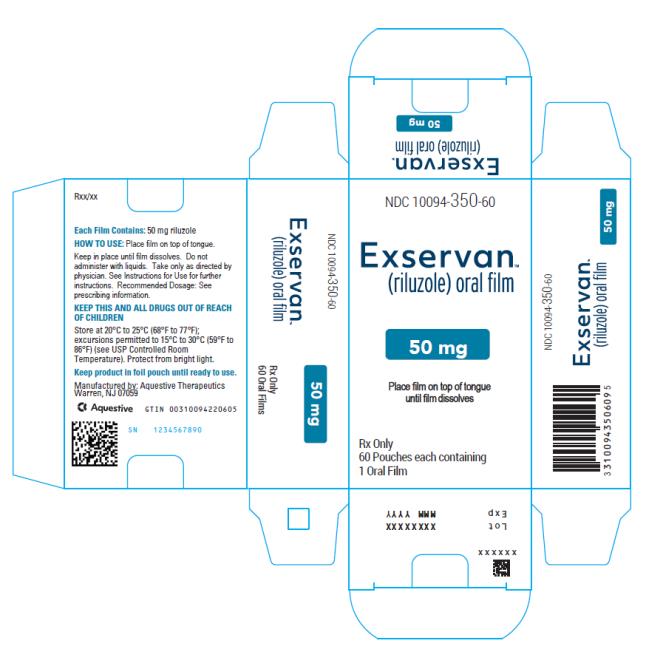
NDC Code(s) : 10094-350-60
Packager : Aquestive Therapeutics
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| EXSERVANriluzole FILM | ||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| LABELER - Aquestive Therapeutics(615102303) |
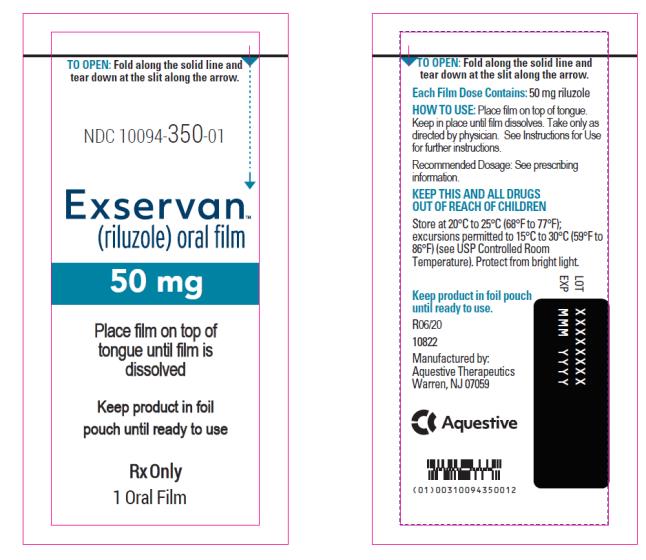
PRINCIPAL DISPLAY PANEL
NDC 10094-350-01
Exservan
(riluzole) oral film
50 mg
Rx Only
1 Oral Film
PRINCIPAL DISPLAY PANEL
NDC 10094-350-60
Exservan
(riluzole) oral film
50 mg
Rx Only
60 Pouches each containing
1 Oral Film