NDC Code(s) : 10157-8407-1, 10157-8407-2, 10157-8407-3, 10157-8407-4, 10157-8407-5
Packager : Blistex Inc.
Category : HUMAN OTC DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Blistex Lip MedexPETROLATUM, MENTHOL, UNSPECIFIED FORM, CAMPHOR (SYNTHETIC), and PHENOL PASTE | ||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
| LABELER - Blistex Inc.(005126354) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Blistex Inc. | 005126354 | MANUFACTURE(10157-8407) | |
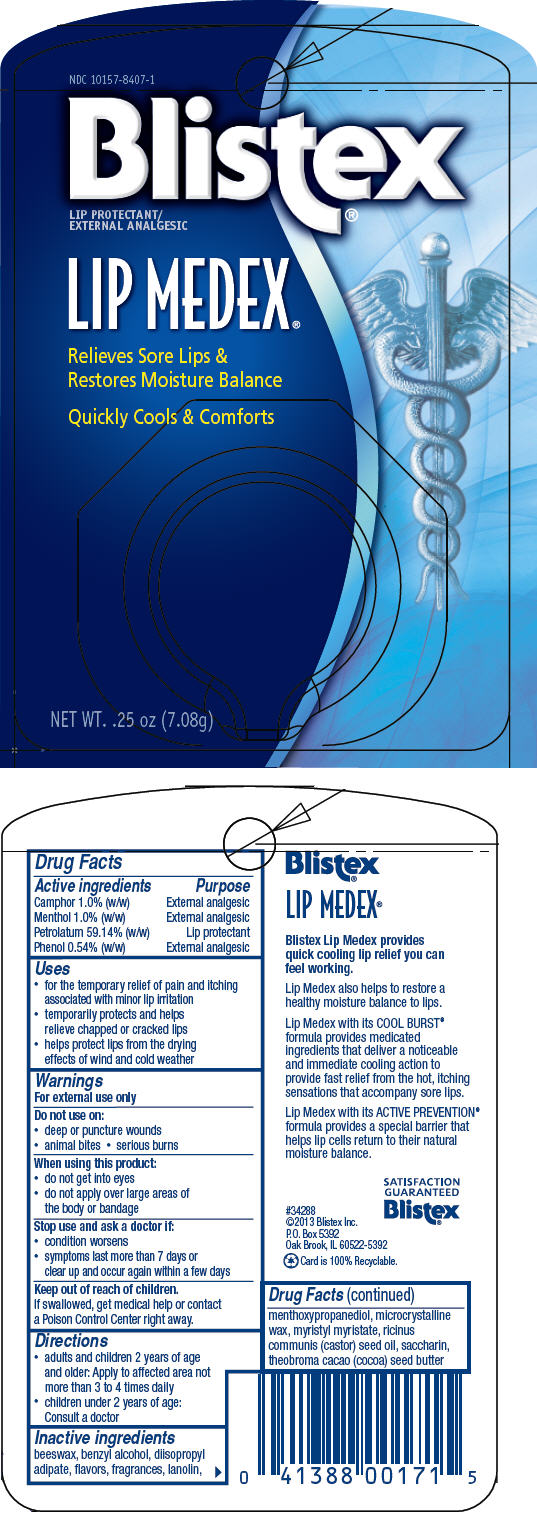
PRINCIPAL DISPLAY PANEL
NDC 10157-8407-1
Blistex®
LIP PROTECTANT/
EXTERNAL ANALGESIC
LIP MEDEX®
Relieves Sore Lips &
Restores Moisture Balance
Quickly Cools & Comforts
NET WT. .25 oz (7.08g)