NDC Code(s) : 10702-025-03, 10702-025-01, 10702-025-10, 10702-029-03, 10702-029-01, 10702-029-10
Packager : KVK-TECH, Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : CIV
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Phentermine HydrochloridePhentermine Hydrochloride TABLET | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Phentermine HydrochloridePhentermine Hydrochloride CAPSULE | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| LABELER - KVK-TECH, Inc.(173360061) |
| REGISTRANT - KVK-Tech, Inc.(173360061) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| KVK-Tech, Inc. | 173360061 | manufacture(10702-025, 10702-029) | |
PRINCIPAL DISPLAY PANEL
Pack Size: 30s
NDC 10702- 029-03
PHENTERMINE HYDROCHLORIDE CAPSULES, USP
37.5 mg
30 CAPSULES
Rx Only
KVK-TECH, INC.

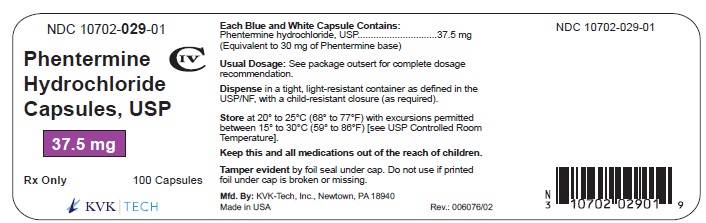
Pack Size: 100s
NDC 10702- 029-01
PHENTERMINE HYDROCHLORIDE CAPSULES, USP
37.5 mg
100 CAPSULES
Rx Only
KVK-TECH, INC.
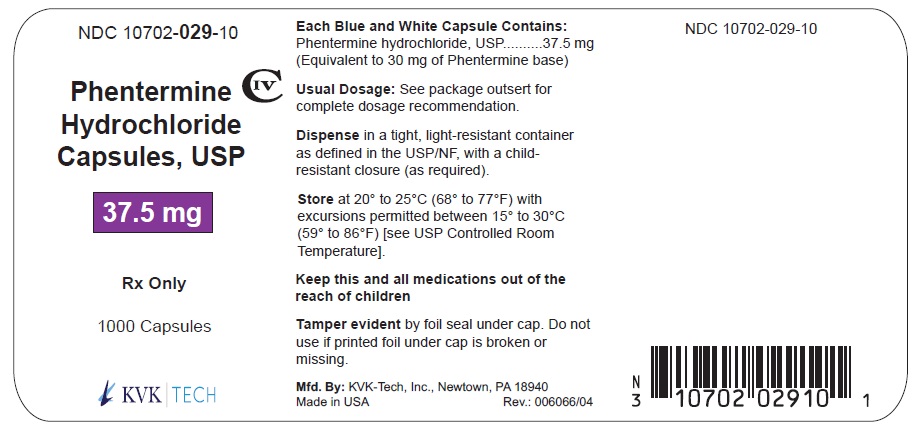
Pack Size: 1000s
NDC 10702- 029-10
PHENTERMINE HYDROCHLORIDE CAPSULES, USP
37.5 mg
1000 CAPSULES
Rx Only
KVK-TECH, INC.
PRINCIPAL DISPLAY PANEL
Pack Size: 30s
NDC 10702- 025-03
PHENTERMINE HYDROCHLORIDE TABLETS, USP
37.5 mg
30 TABLETS
Rx Only
KVK-TECH, INC.

Pack Size: 100s
NDC 10702- 025-01
PHENTERMINE HYDROCHLORIDE TABLETS, USP
37.5 mg
100 TABLETS
Rx Only
KVK-TECH, INC.

Pack Size: 1000s
NDC 10702- 025-10
PHENTERMINE HYDROCHLORIDE TABLETS, USP
37.5 mg
1000 TABLETS
Rx Only
KVK-TECH, INC.










