NDC Code(s) : 10702-183-50, 10702-171-03
Packager : KVK-Tech, Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : CII
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Oxycodone HydrochlorideOxycodone Hydrochloride SOLUTION | ||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| OXYCODONE HYDROCHLORIDEoxycodone hydrochloride SOLUTION | ||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| LABELER - KVK-Tech, Inc.(173360061) |
| REGISTRANT - ABHAI, LLC.(079385868) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| KVK-Tech, Inc. | 173360061 | manufacture(10702-183, 10702-171) | |
PRINCIPAL DISPLAY PANEL
NDC 10702- 183-50
Oxycodone Hydrochloride Oral Solution, USP CII
5 mg per 5 mL
(1 mg per 1 mL)
Pharmacist: Dispense the accompnying Medication Guide to each patient
SUGAR FREE . ALCOHOL FREE
R X Only 500 mL
KVK-TECH, INC.

PRINCIPAL DISPLAY PANEL
NDC 10702- 171-03
Oxycodone Hydrochloride Oral Solution, USP CII
100 mg per 5 mL
(20 mg per 1 mL)
Pharmacist: Dispense the accompnying Medication Guide to each patients.
ONLY FOR USE IN PATIENTS WHO ARE OPIOID TOLERANT
SUGAR FREE . ALCOHOL FREE
R X Only 30 mL
KVK-TECH, INC.

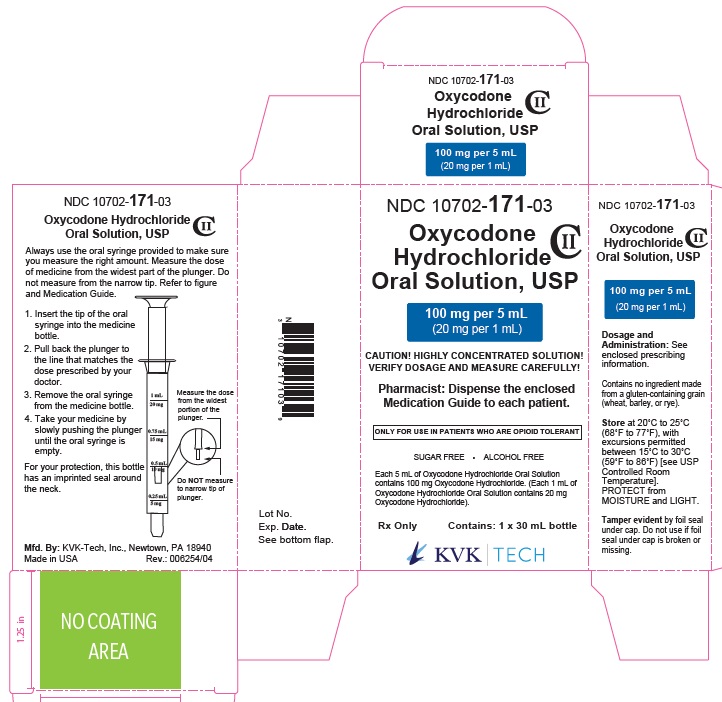
PRINCIPAL DISPLAY PANEL
NDC 10702- 171-03
Oxycodone Hydrochloride Oral Solution, USP CII
100 mg per 5 mL
(20 mg per 1 mL)
CAUTION! HIGHLY CONCENTRATED SOLUTION!
VERIFY DOSAGE AND MEASURE CAREFULLY!
Pharmacist: Dispense the enclosed Medication Guide to each patients.
ONLY FOR USE IN PATIENTS WHO ARE OPIOID TOLERANT
SUGAR FREE . ALCOHOL FREE
Each 5 mL of Oxycodone Hydrochloride Oral Solution contains 100 mg Oxycodone Hydrochloride. (Each 1 mL of Oxycodone Hydrochloride Oral Solution contains 20 mg Oxycodone Hydrochloride).
R X only Containes: 1 X 30 mL bottle
KVK-TECH, INC.
PRINCIPAL DISPLAY PANEL
NDC 10702- 183-50
Oxycodone Hydrochloride Oral Solution, USP CII
5 mg per 5 mL
(1 mg per 1 mL)
Pharmacist: Dispense the enclosed Medication Guide to each patient.
SUGAR FREE . ALCOHOL FREE
R X only
Contains: 1 X 500 mL bottle
KVK-TECH, INC.










