NDC Code(s) : 10812-091-01, 10812-092-01, 10812-093-01, 10812-094-01, 10812-095-01, 10812-096-01, 10812-097-01, 10812-098-01
Packager : Johnson & Johnson Consumer Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Neutrogena Healthy Skin Compact Makeup Avobenzone, Homosalate, Octisalate, Octocrylene, and Oxybenzone CREAM | ||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| Neutrogena Healthy Skin Compact Makeup Avobenzone, Homosalate, Octisalate, Octocrylene, and Oxybenzone CREAM | ||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| Neutrogena Healthy Skin Compact Makeup Avobenzone, Homosalate, Octisalate, Octocrylene, and Oxybenzone CREAM | ||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| Neutrogena Healthy Skin Compact Makeup Avobenzone, Homosalate, Octisalate, Octocrylene, and Oxybenzone CREAM | ||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| Neutrogena Healthy Skin Compact Makeup Avobenzone, Homosalate, Octisalate, Octocrylene, and Oxybenzone CREAM | ||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| Neutrogena Healthy Skin Compact Makeup Avobenzone, Homosalate, Octisalate, Octocrylene, and Oxybenzone CREAM | ||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| Neutrogena Healthy Skin Compact Makeup Avobenzone, Homosalate, Octisalate, Octocrylene, and Oxybenzone CREAM | ||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| Neutrogena Healthy Skin Compact Makeup Avobenzone, Homosalate, Octisalate, Octocrylene, and Oxybenzone CREAM | ||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
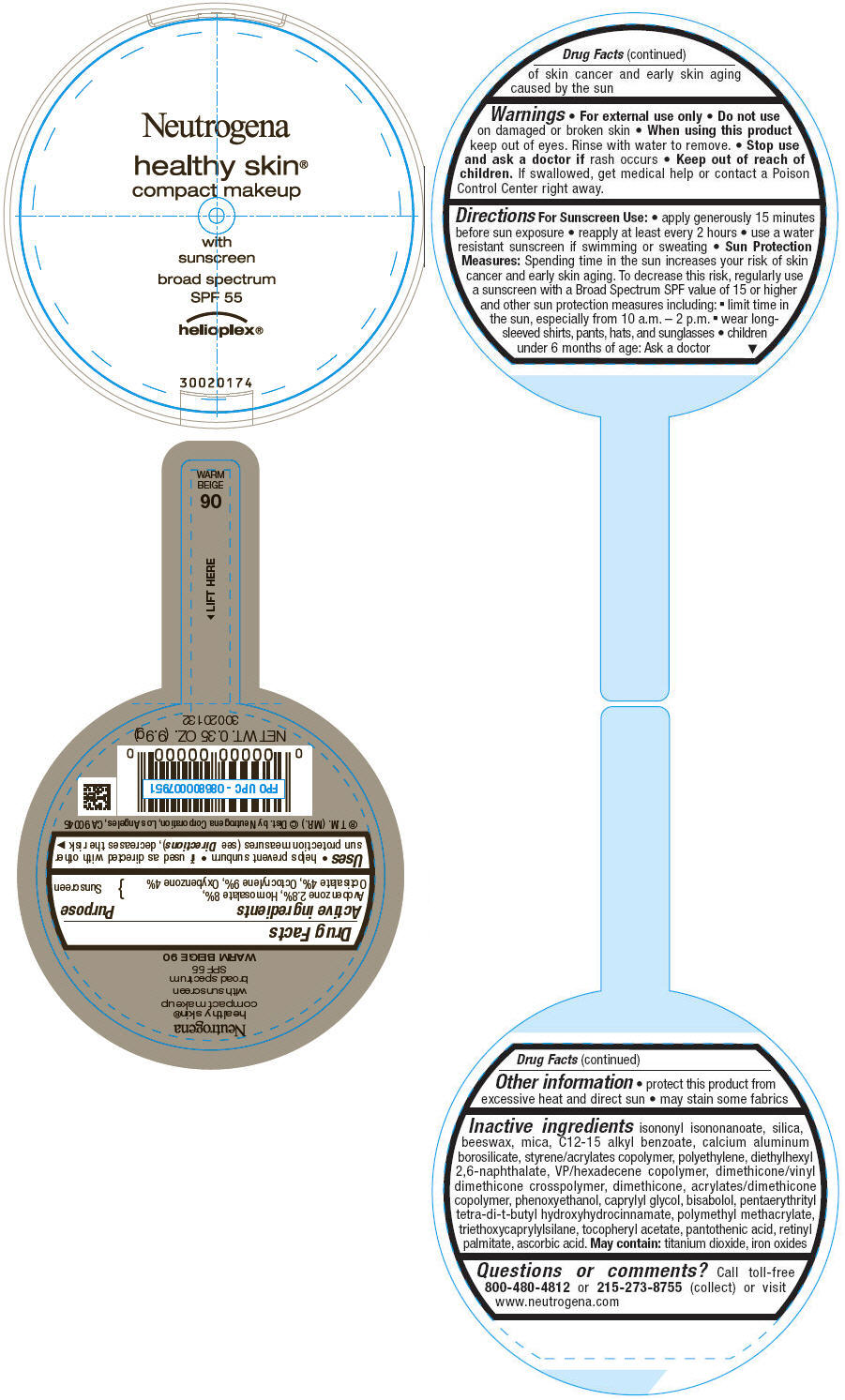
PRINCIPAL DISPLAY PANEL
Neutrogena
healthy skin®
compact makeup
with
sunscreen
broad spectrum
SPF 55
helioplex®
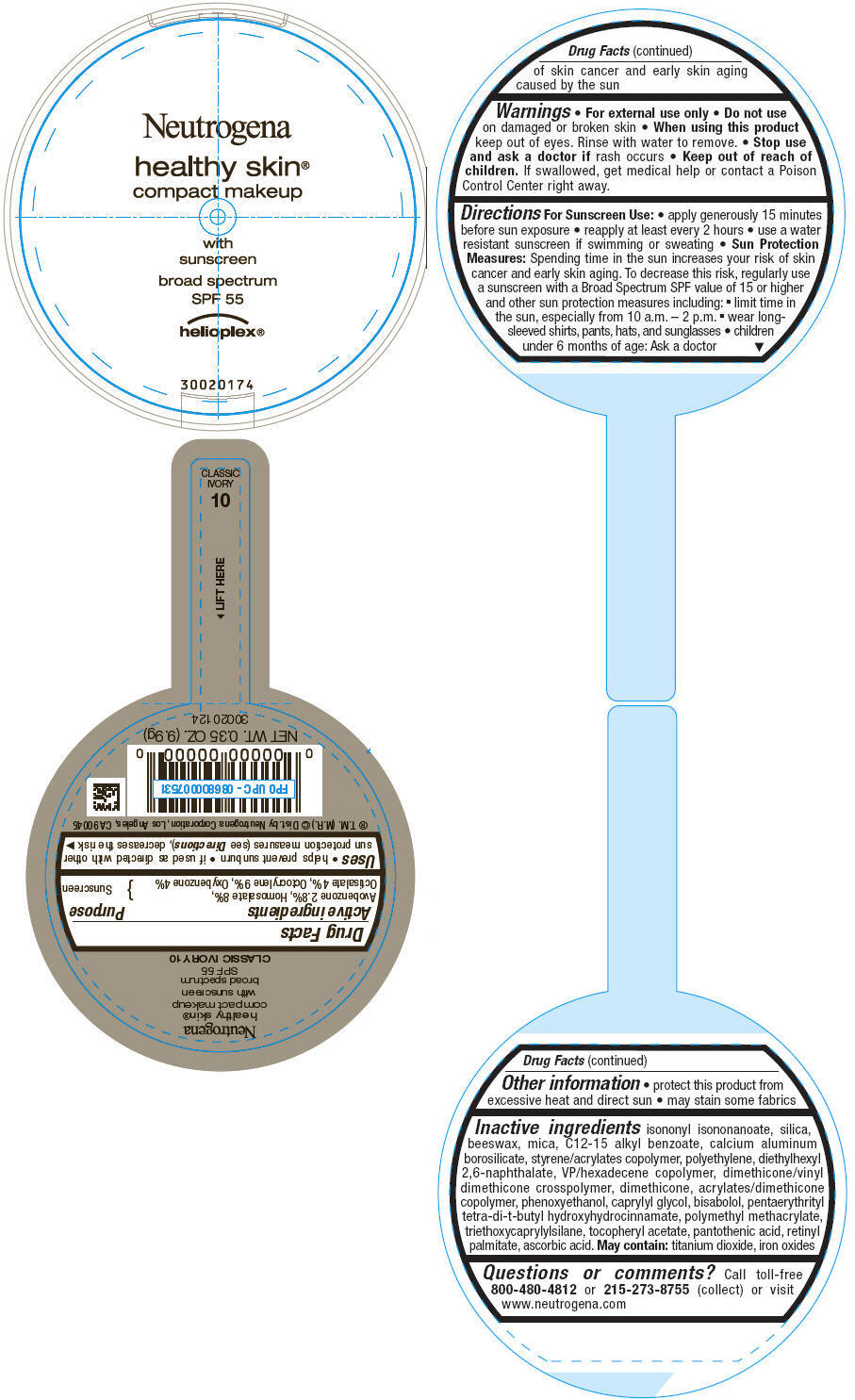
PRINCIPAL DISPLAY PANEL
Neutrogena
healthy skin®
compact makeup
with
sunscreen
broad spectrum
SPF 55
helioplex®
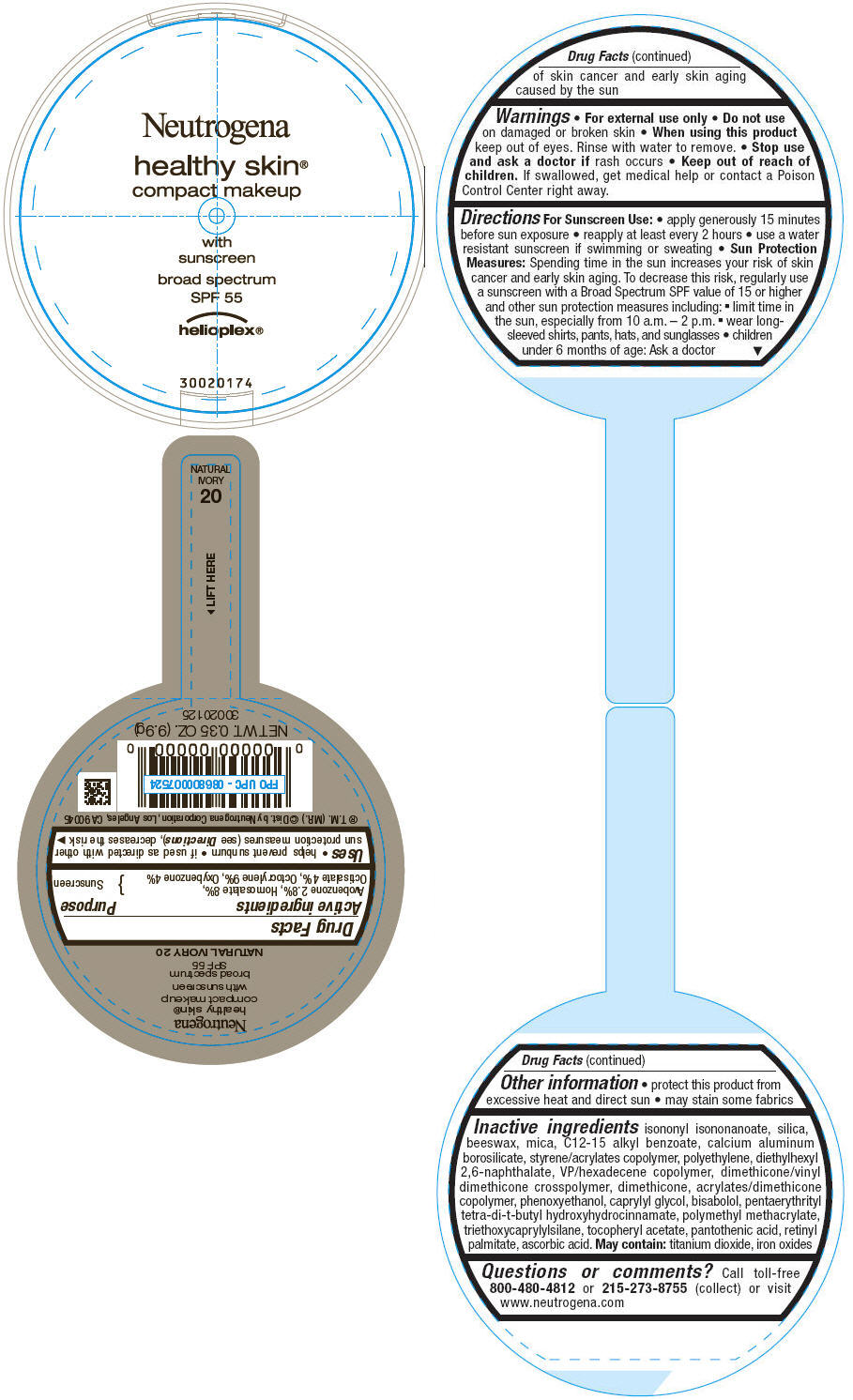
PRINCIPAL DISPLAY PANEL
Neutrogena
healthy skin®
compact makeup
with
sunscreen
broad spectrum
SPF 55
helioplex®
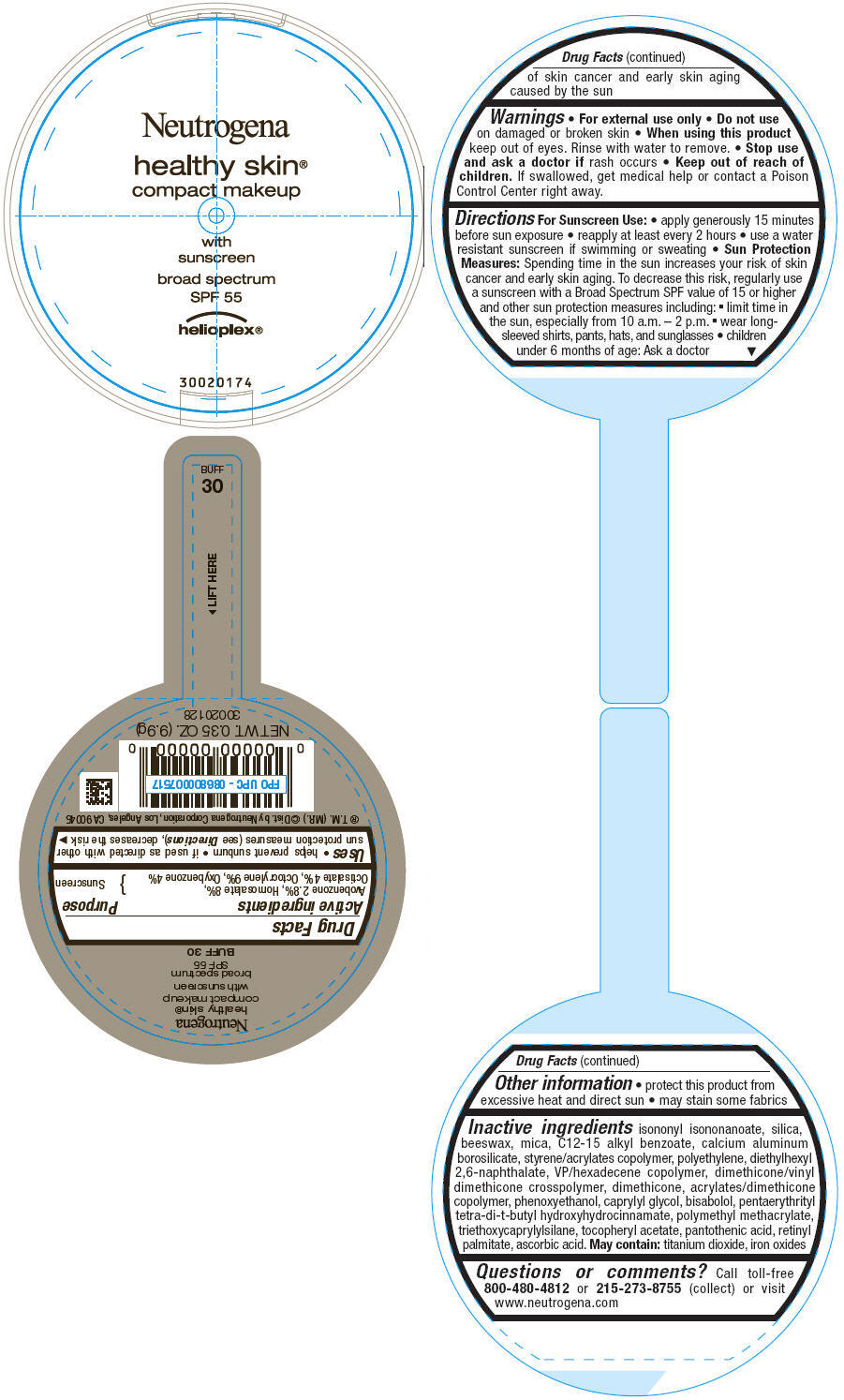
PRINCIPAL DISPLAY PANEL
Neutrogena
healthy skin®
compact makeup
with
sunscreen
broad spectrum
SPF 55
helioplex®
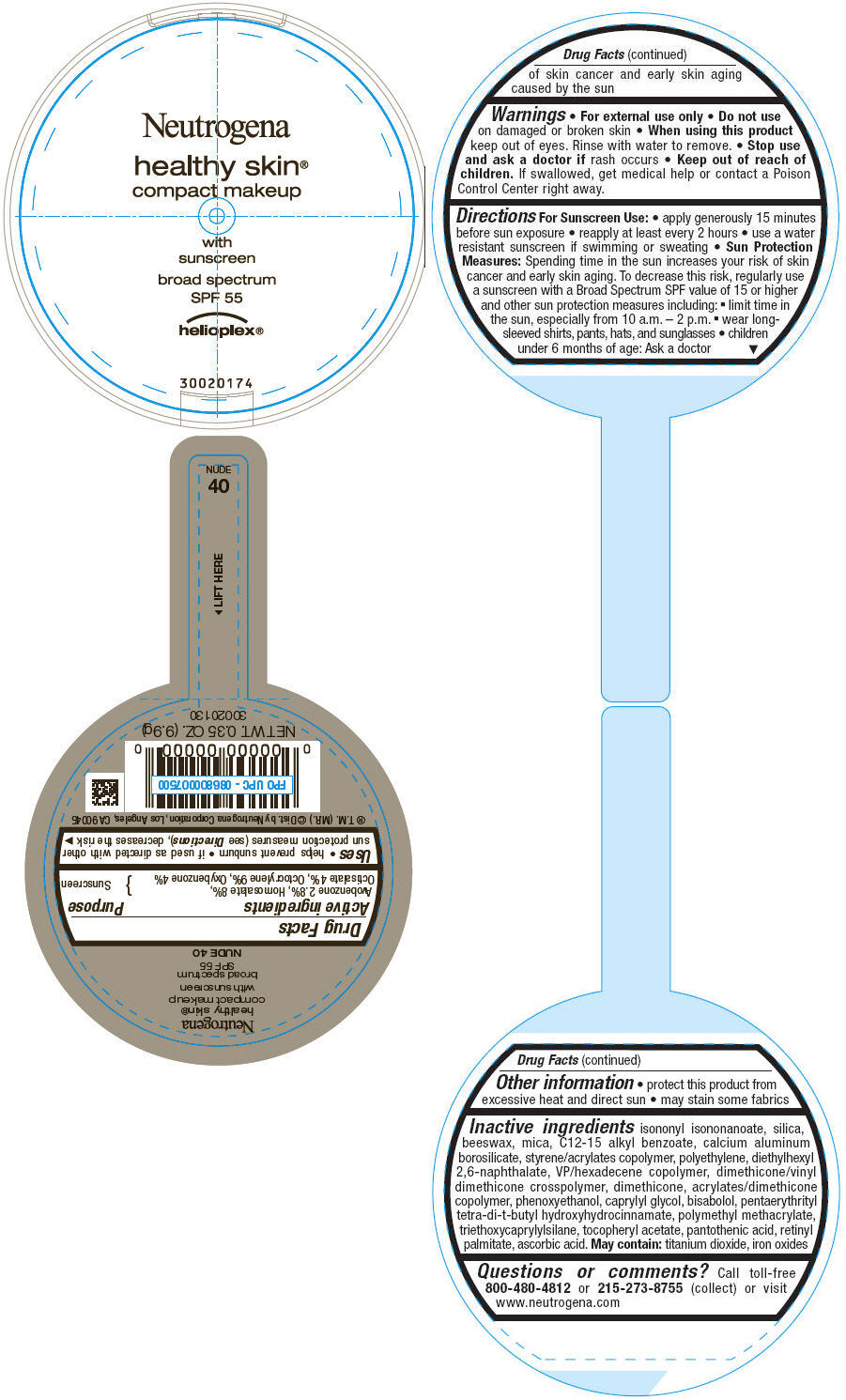
PRINCIPAL DISPLAY PANEL
Neutrogena
healthy skin®
compact makeup
with
sunscreen
broad spectrum
SPF 55
helioplex®
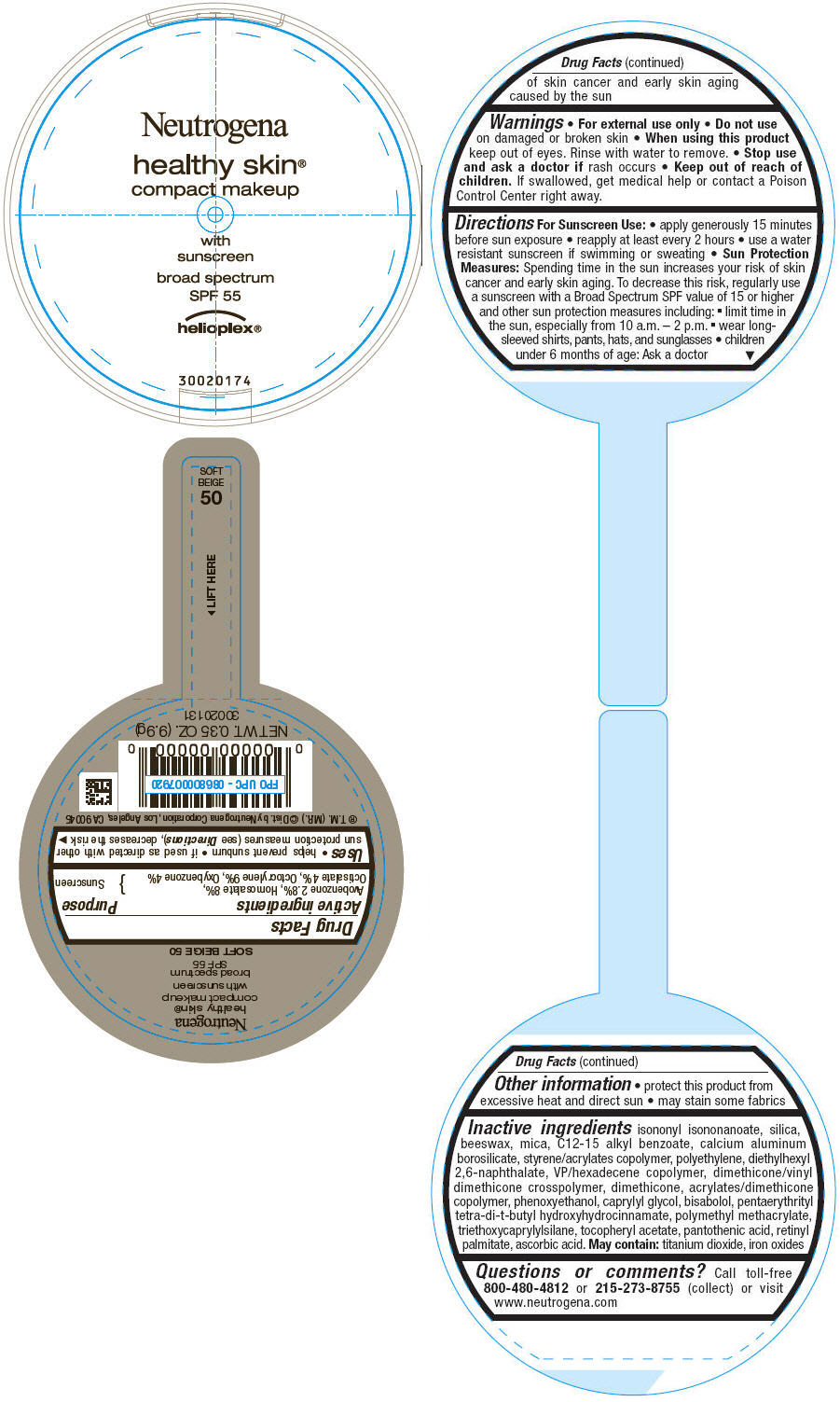
PRINCIPAL DISPLAY PANEL
Neutrogena
healthy skin®
compact makeup
with
sunscreen
broad spectrum
SPF 55
helioplex®
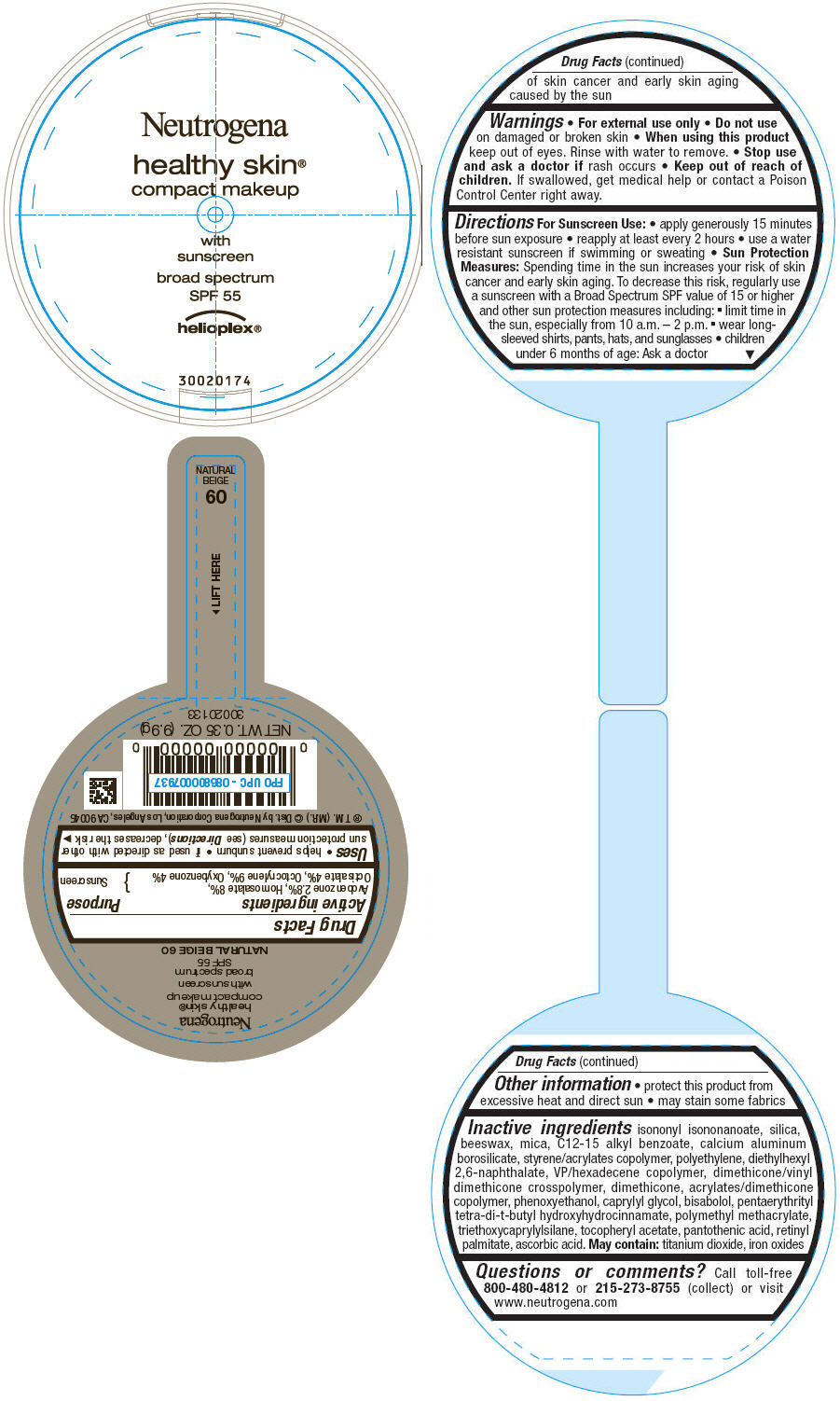
PRINCIPAL DISPLAY PANEL
Neutrogena
healthy skin®
compact makeup
with
sunscreen
broad spectrum
SPF 55
helioplex®
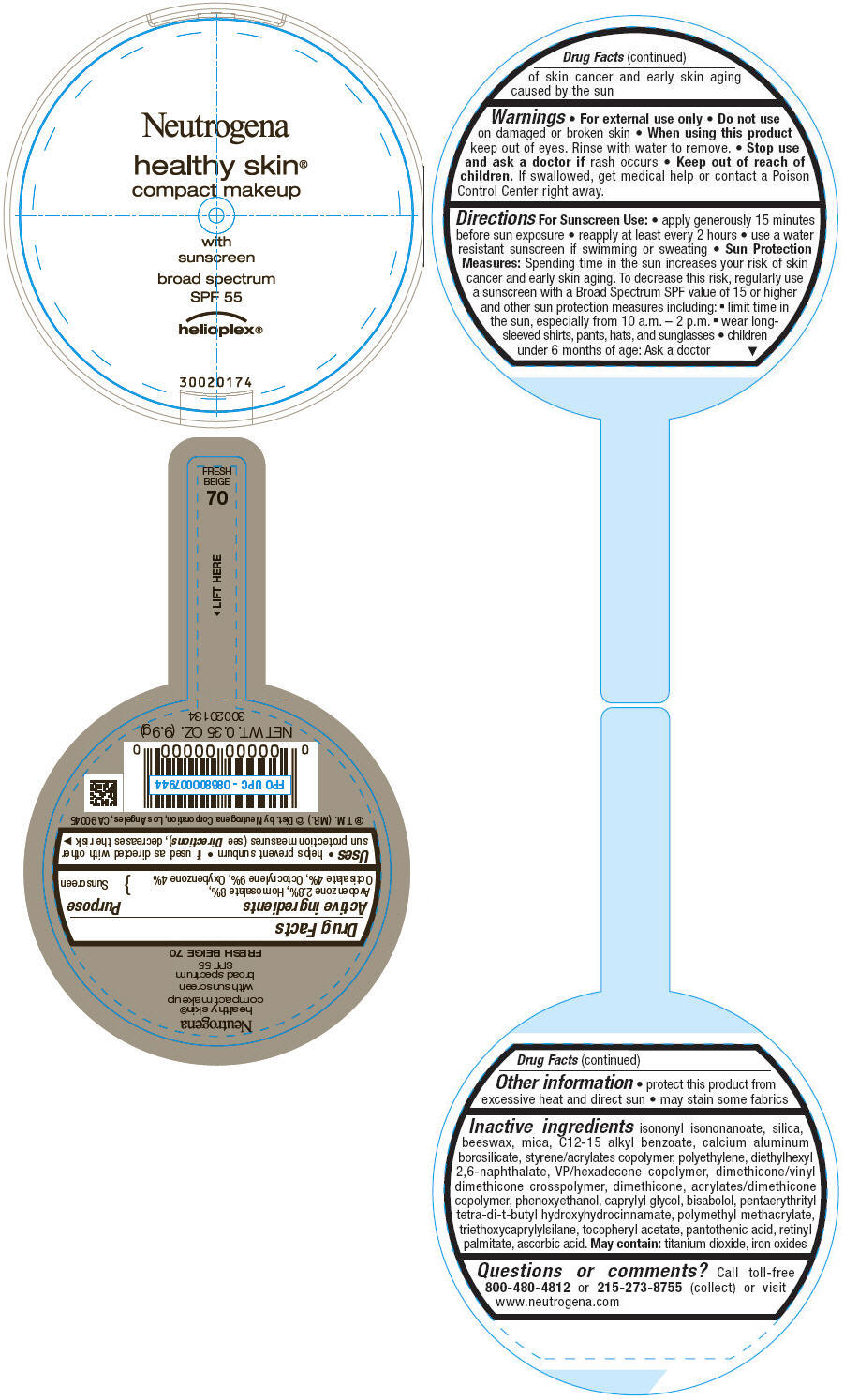
PRINCIPAL DISPLAY PANEL
Neutrogena
healthy skin®
compact makeup
with
sunscreen
broad spectrum
SPF 55
helioplex®