NDC Code(s) : 10812-153-01
Packager : Johnson & Johnson Consumer Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Neutrogena Rapid Clear Acne Defense FaceSalicylic Acid LOTION | ||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
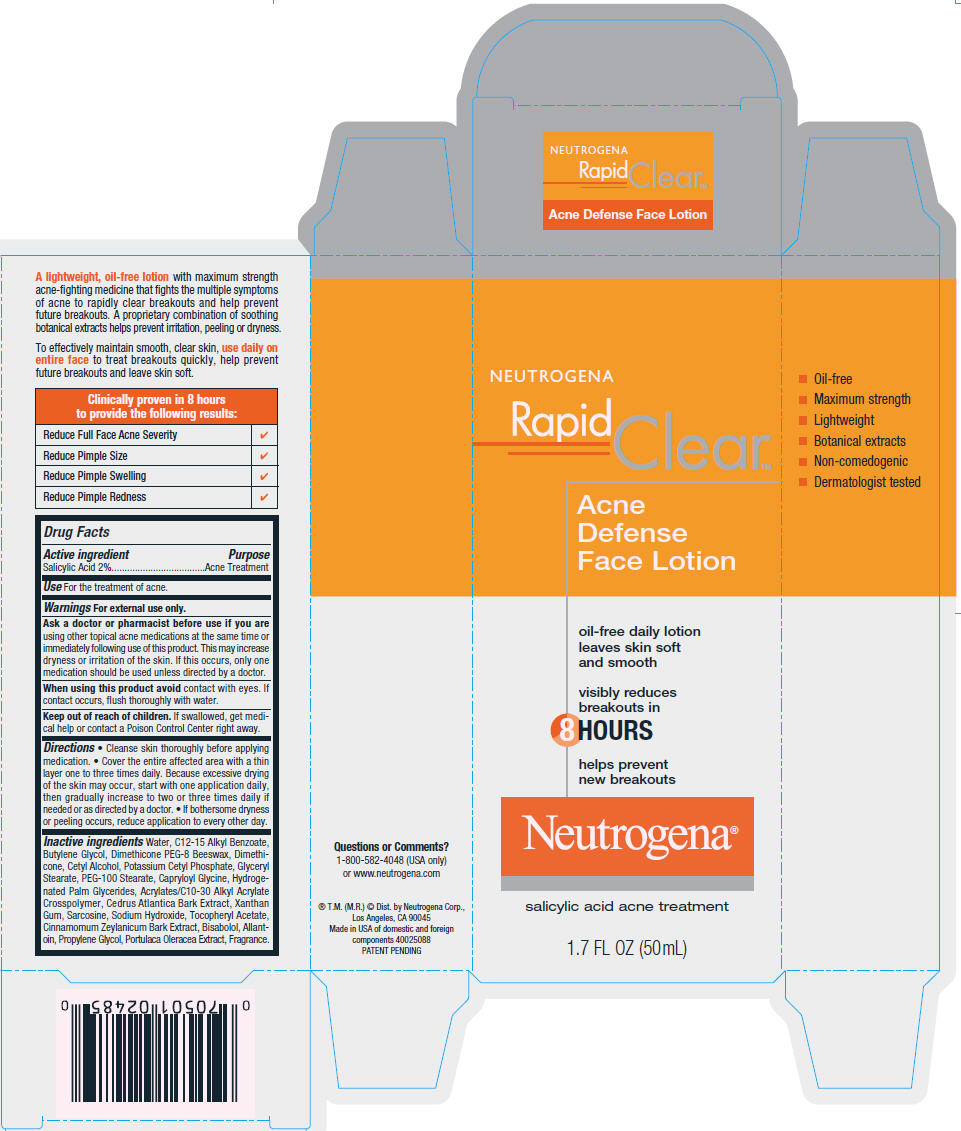
PRINCIPAL DISPLAY PANEL
NEUTROGENA
Rapid
Clear™
Acne
Defense
Face Lotion
oil-free daily lotion
leaves skin soft
and smooth
visibly reduces
breakouts in
8 HOURS
helps prevent
new breakouts
Neutrogena®
salicylic acid acne treatment
1.7 FL OZ (50mL)