NDC Code(s) : 10812-457-01, 10812-459-01, 10812-456-01, 10812-458-01
Packager : Johnson & Johnson Consumer Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Neutrogena Healthy Skin Brightening Eye Perfector Titanium Dioxide CREAM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Neutrogena Healthy Skin Brightening Eye Perfector Titanium Dioxide CREAM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Neutrogena Healthy Skin Brightening Eye Perfector Titanium Dioxide CREAM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Neutrogena Healthy Skin Brightening Eye Perfector Titanium Dioxide CREAM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
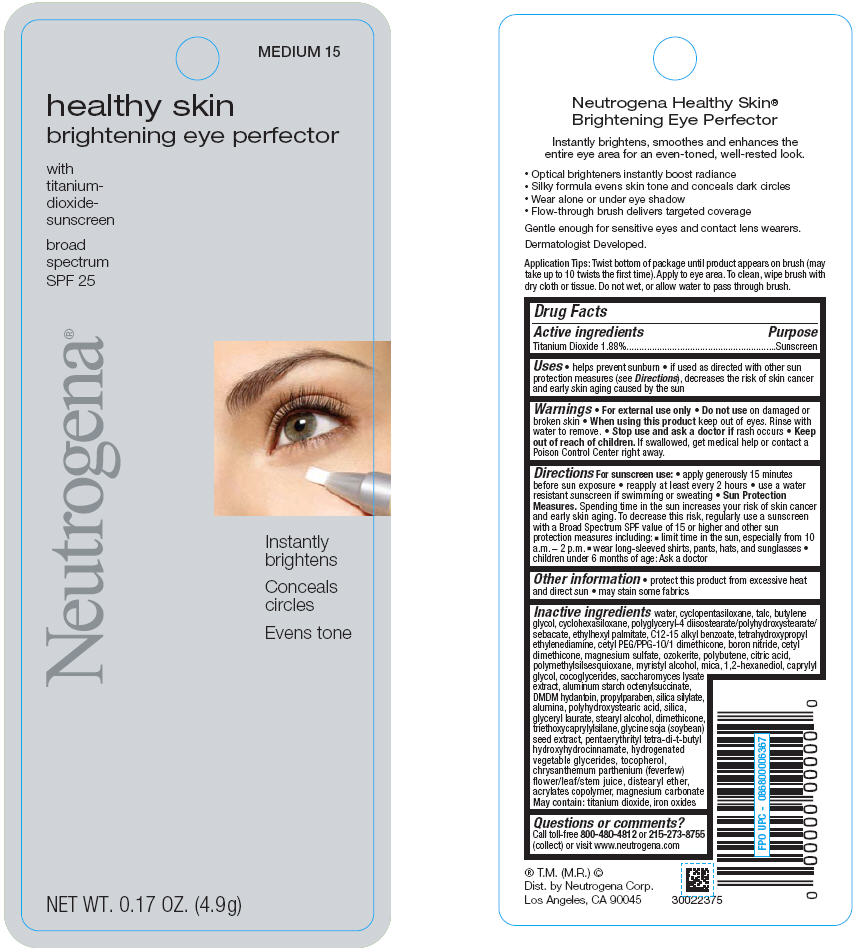
PRINCIPAL DISPLAY PANEL
LIGHT 10
healthy skin
brightening eye perfector
with
titanium-
dioxide-
sunscreen
broad
spectrum
SPF 25
Neutrogena®
Instantly
brightens
Conceals
circles
Evens tone
NET WT. 0.17 OZ. (4.9g)
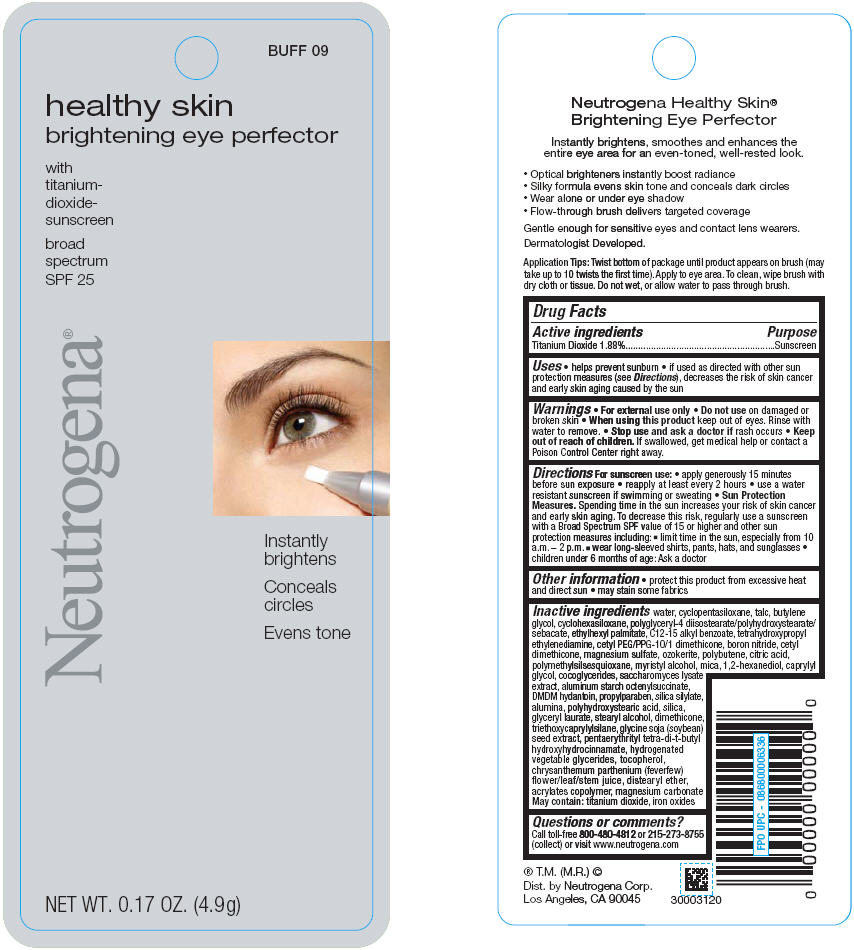
PRINCIPAL DISPLAY PANEL
BUFF 09
healthy skin
brightening eye perfector
with
titanium-
dioxide-
sunscreen
broad
spectrum
SPF 25
Neutrogena®
Instantly
brightens
Conceals
circles
Evens tone
NET WT. 0.17 OZ. (4.9g)
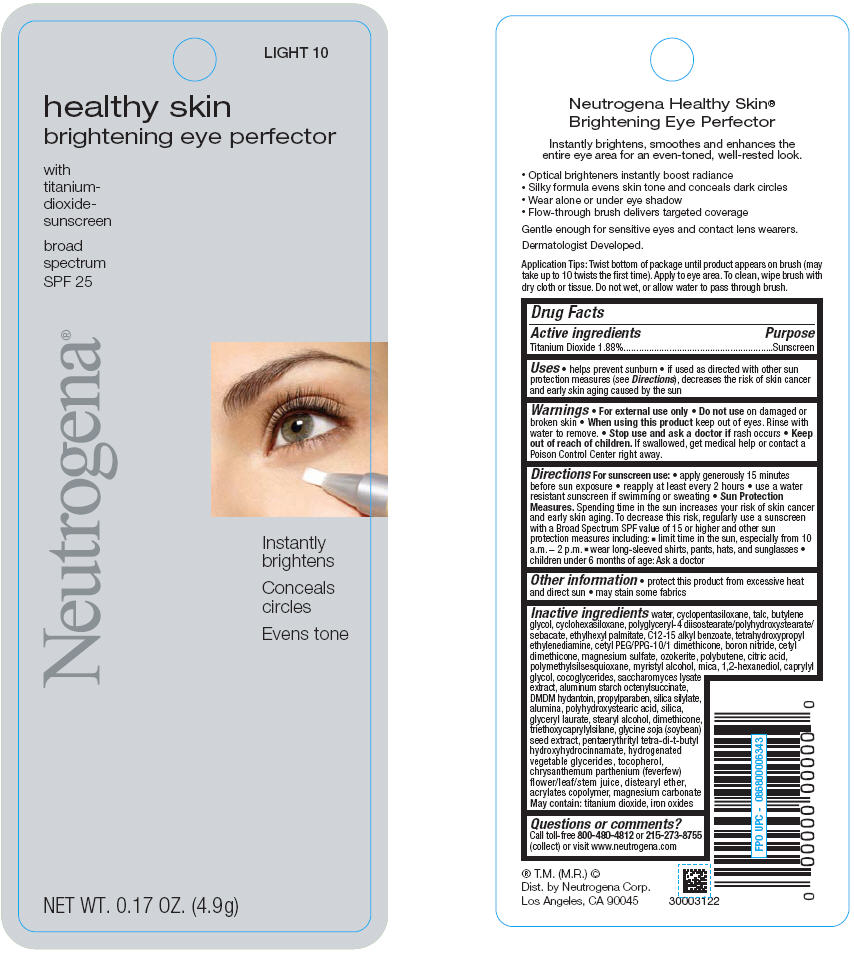
PRINCIPAL DISPLAY PANEL
FAIR 05
healthy skin
brightening eye perfector
with
titanium-
dioxide-
sunscreen
broad
spectrum
SPF 25
Neutrogena®
Instantly
brightens
Conceals
circles
Evens tone
NET WT. 0.17 OZ. (4.9g)
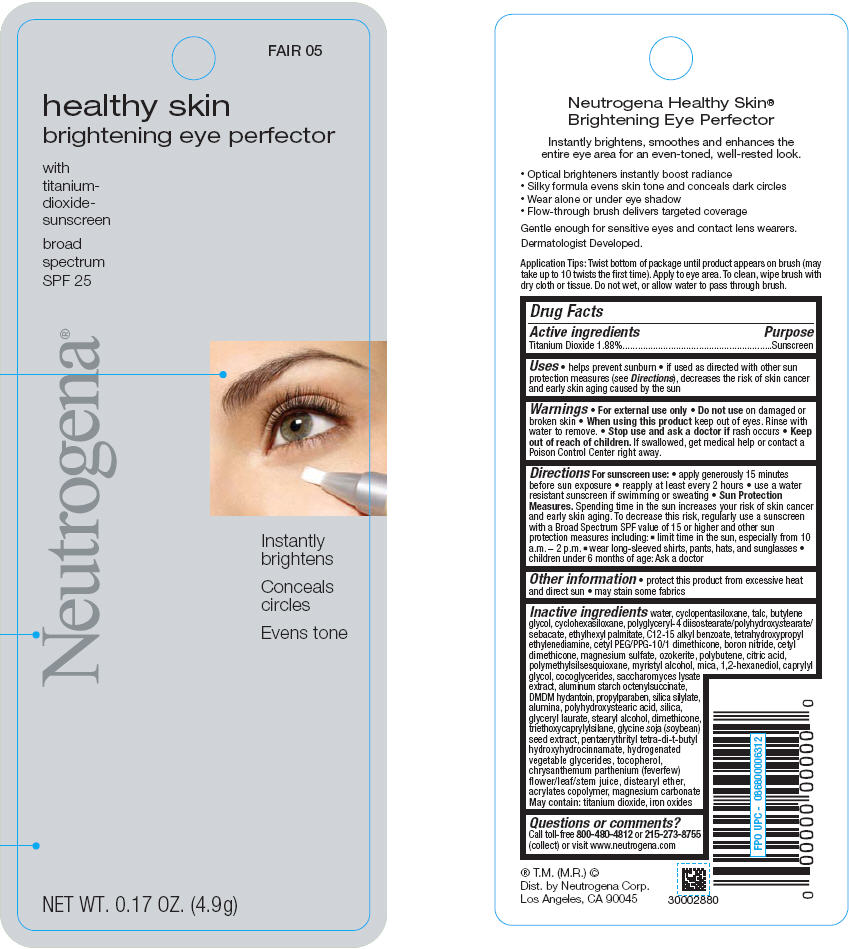
PRINCIPAL DISPLAY PANEL
MEDIUM 15
healthy skin
brightening eye perfector
with
titanium-
dioxide-
sunscreen
broad
spectrum
SPF 25
Neutrogena®
Instantly
brightens
Conceals
circles
Evens tone
NET WT. 0.17 OZ. (4.9g)