NDC Code(s) : 10812-702-01
Packager : Johnson & Johnson Consumer Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Neutrogena Wet Skin Swim Humidity Sweat Avobenzone, Homosalate, Octisalate, Octocrylene, and Oxybenzone AEROSOL, SPRAY | |||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
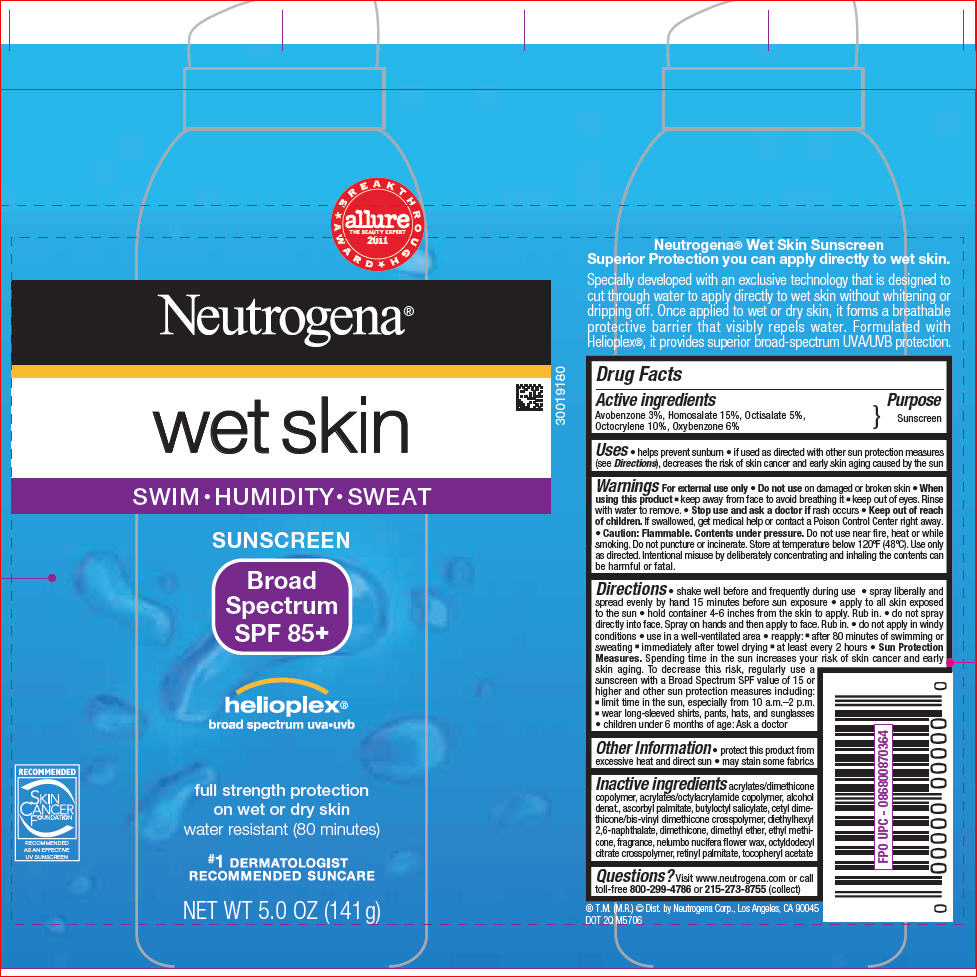
PRINCIPAL DISPLAY PANEL
BREAKTHROUGH AWARD
allure
THE BEAUTY EXPERT
2011
Neutrogena®
wet skin
SWIM•HUMIDITY•SWEAT
SUNSCREEN
Broad
Spectrum
SPF 85+
helioplex®
broad spectrum uva•uvb
full strength protection
on wet or dry skin
water resistant (80 minutes)
#1 DERMATOLOGIST
RECOMMENDED SUNCARE
NET WT 5.0 OZ (141 g)