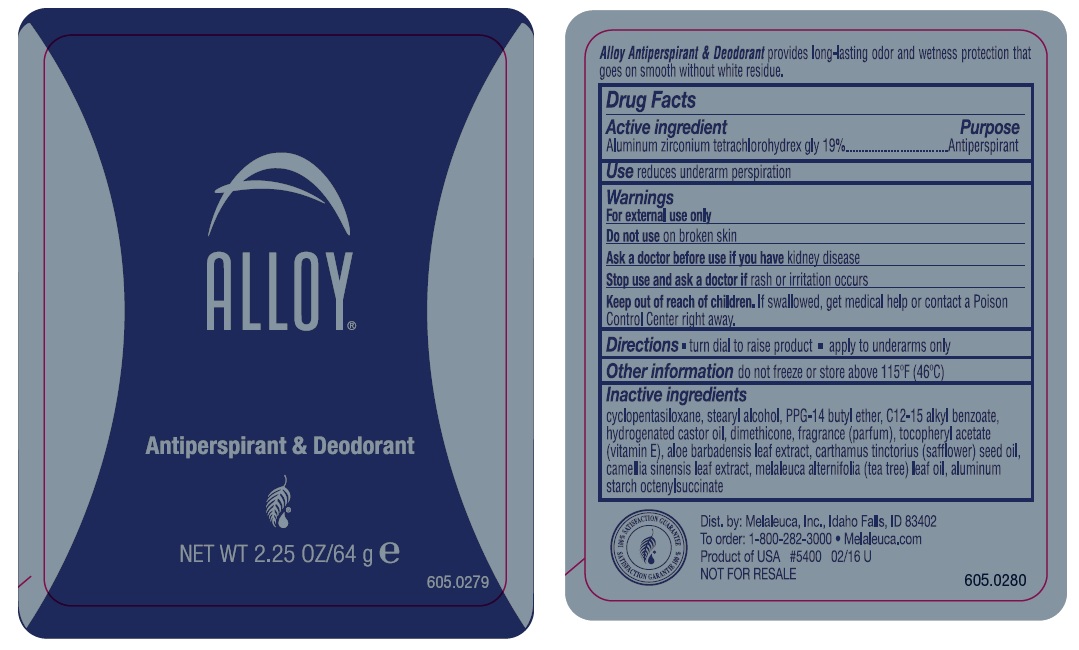
NDC Code(s) : 10889-421-01
Packager : VVF Kansas Services LLC
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Melaleuca Alloy OriginalAluminum Zirconium Tetrachlorohydrex GLY STICK | ||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
PRINCIPAL DISPLAY PANEL