NDC Code(s) : 11444-001-03, 11444-001-04, 11444-001-06
Packager : W. F. Young, Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Absorbine Jr.Menthol LIQUID | |||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
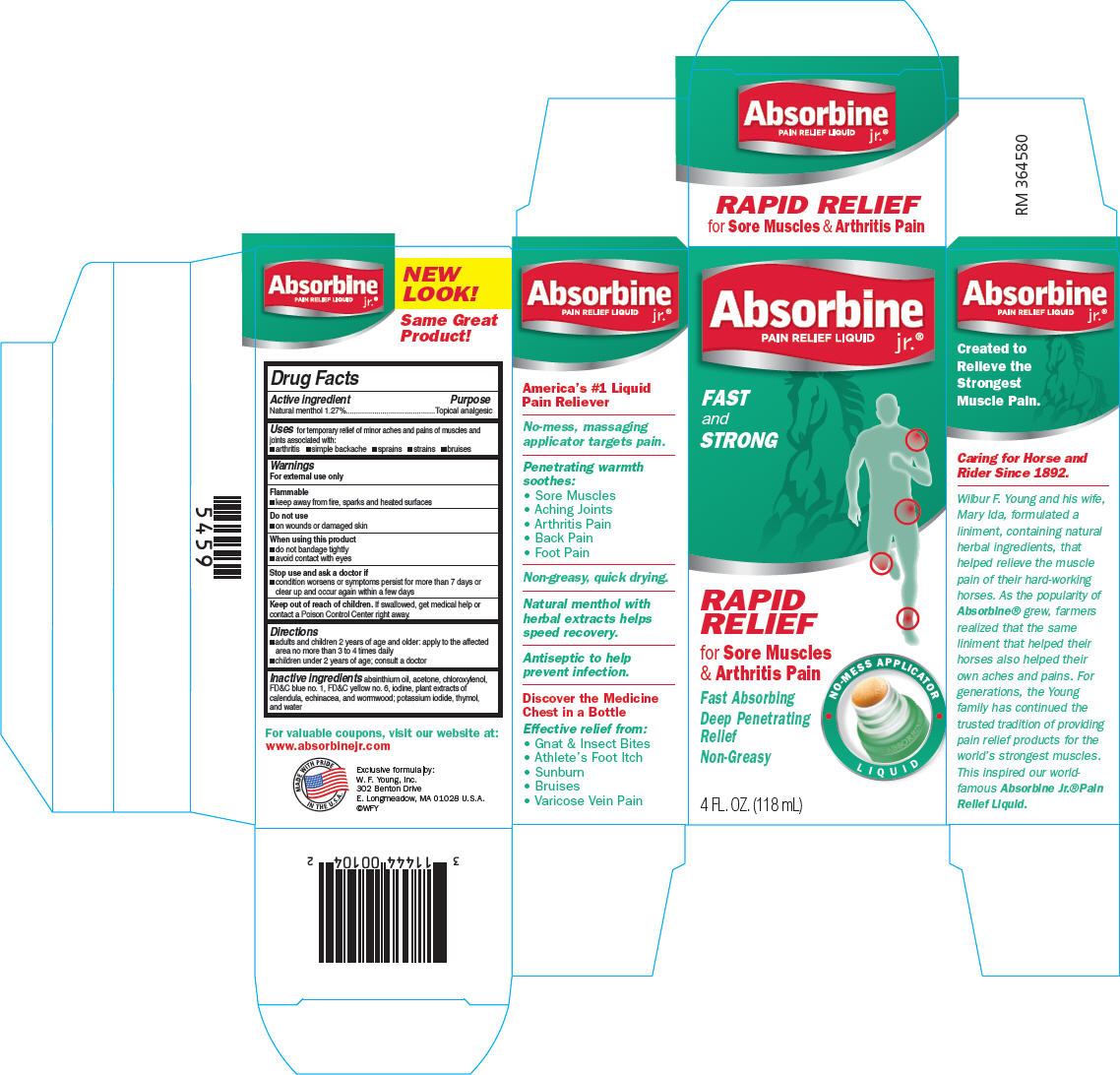
PRINCIPAL DISPLAY PANEL
Absorbine jr.®
PAIN RELIEF LIQUID
FAST
and
STRONG
RAPID
RELIEF
for Sore Muscles
& Arthritis Pain
Fast Absorbing
Deep Penetrating
Relief
Non-Greasy
NO-MESS APPLICATOR
LIQUID
4 FL. OZ. (118 mL)