NDC Code(s) : 11523-7291-1
Packager : MSD Consumer Care, Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Coppertone avobenzone, homosalate, octisalate, and octocrylene LOTION | ||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
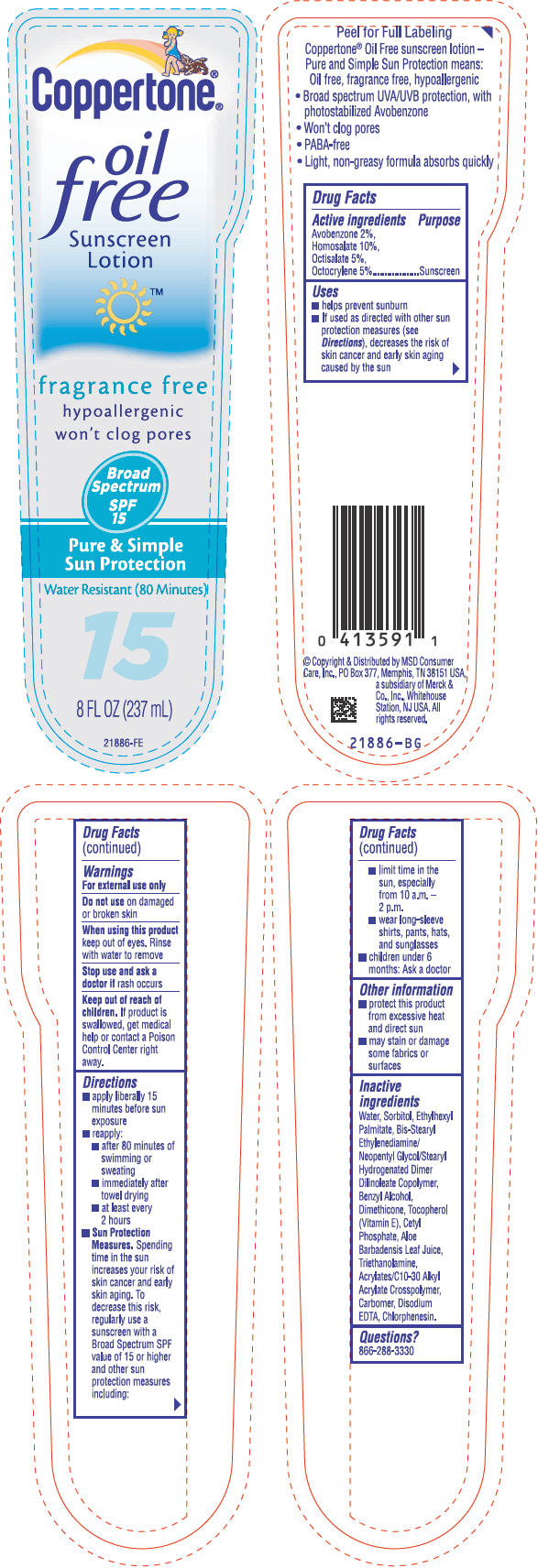
PRINCIPAL DISPLAY PANEL
®
Coppertone®
oil
free
Sunscreen
Lotion
™
fragrance free
hypoallergenic
won't clog pores
Broad
Spectrum
SPF
15
Pure & Simple
Sun Protection
Water Resistant (80 Minutes)
15
8 FL OZ (237 mL)
21886-FE