NDC Code(s) : 11523-7367-1
Packager : Bayer HealthCare LLC.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Coppertone Clearly Sheer For Sunny Days Avobenzone, homosalate, octisalate, and octocrylene LOTION | ||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
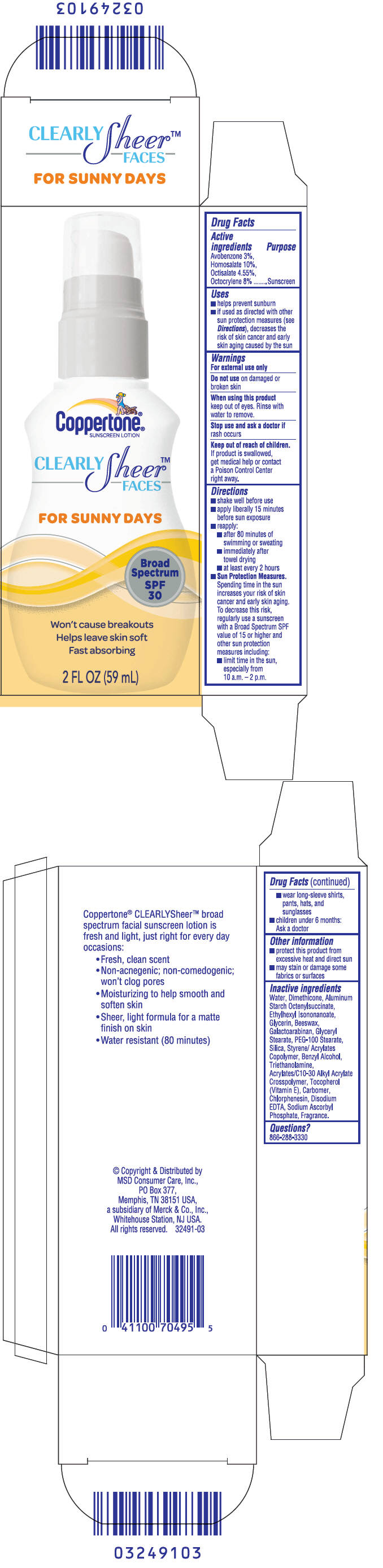
PRINCIPAL DISPLAY PANEL
Coppertone
®
SUNSCREEN LOTION
CLEARLY
Sheer
™
FACES
FOR SUNNY DAYS
Broad
Spectrum
SPF
30
Won't cause breakouts
Helps leave skin soft
Fast absorbing
2 FL OZ (59 mL)