NDC Code(s) : 11704-270-01
Packager : Meridian Medical Technologies, Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Cyanokit hydroxocobalamin INJECTION, POWDER, LYOPHILIZED, FOR SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
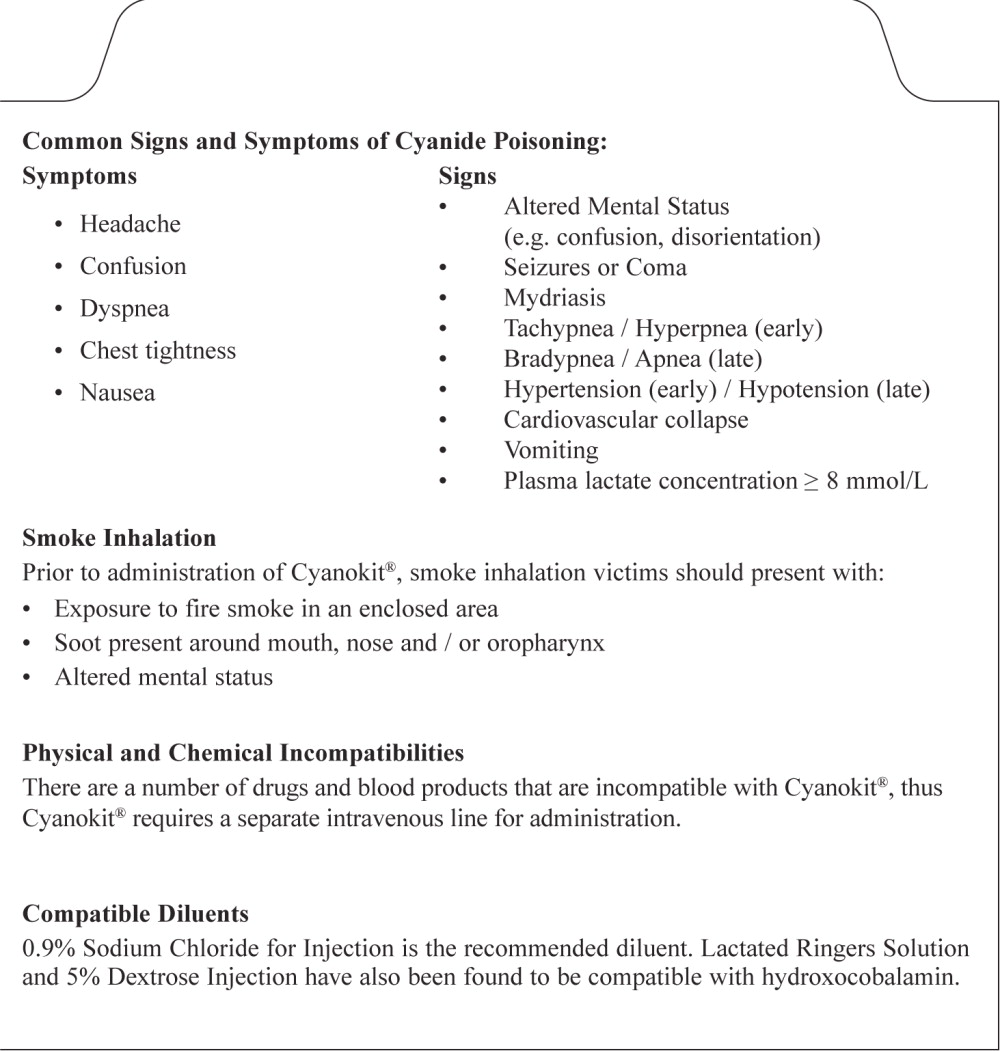
PRINCIPAL DISPLAY PANEL
PACKAGE LABEL – PRINCIPAL DISPLAY PANEL – 5 g BOTTLE LABEL
Cyanokit ® 5 g
Hydroxocobalamin for Injection
(2.5 g per vial)
For Intravenous Use
To be reconstituted with 100 mL
per vial of 0.9% Sodium Chloride Injection
Diluent Not Included
Rx Only
Manufactured by:
Merck Santé s.a.s.,
Semoy, France
Distributed by
Meridian Medical TechnologiesTM, Inc.
Columbia, MD 21046
A wholly-owned subsidiary of King Pharmaceuticals®, Inc.
1-800-776-3637
MERIDIAN
MEDICAL TECHNOLOGIES™
PRINCIPAL DISPLAY PANEL
PACKAGE LABEL – PRINCIPAL DISPLAY PANEL – 5 g INNER CARTON
Cyanokit ® 5 g
Hydroxocobalamin for Injection
(2.5 g per vial)
For Intravenous Use
To be reconstituted with 100 mL
per vial of 0.9% Sodium Chloride Injection
Diluent Not Included
Rx Only
Manufactured by:
Merck Santé s.a.s.,
Semoy, France
Distributed by
Meridian Medical TechnologiesTM, Inc.
Columbia, MD 21046
A wholly-owned subsidiary of King Pharmaceuticals®, Inc.
1-800-776-3637
MERIDIAN
MEDICAL TECHNOLOGIES™

PRINCIPAL DISPLAY PANEL
PACKAGE LABEL – PRINCIPAL DISPLAY PANEL – 5 g OUTER CARTON
Cyanokit ® 5 g
Hydroxocobalamin for Injection
(2.5 g per vial)
For Intravenous Use
To be reconstituted with 100 mL
per vial of 0.9% Sodium Chloride Injection
Diluent Not Included
Rx Only
Manufactured by:
Merck Santé s.a.s.,
Semoy, France
Distributed by
Meridian Medical TechnologiesTM, Inc.
Columbia, MD 21046
A wholly-owned subsidiary of King Pharmaceuticals®, Inc.
1-800-776-3637
MERIDIAN
MEDICAL TECHNOLOGIES™

PRINCIPAL DISPLAY PANEL
PACKAGE LABEL – PRINCIPAL DISPLAY PANEL – 5 g PATIENT INSTRUCTION CARD SIDE A
Cyanokit ® 5 g
Hydroxocobalamin for Injection
(2.5 g per vial)
For Intravenous Use
To be reconstituted with 100 mL
per vial of 0.9% Sodium Chloride Injection
Diluent Not Included
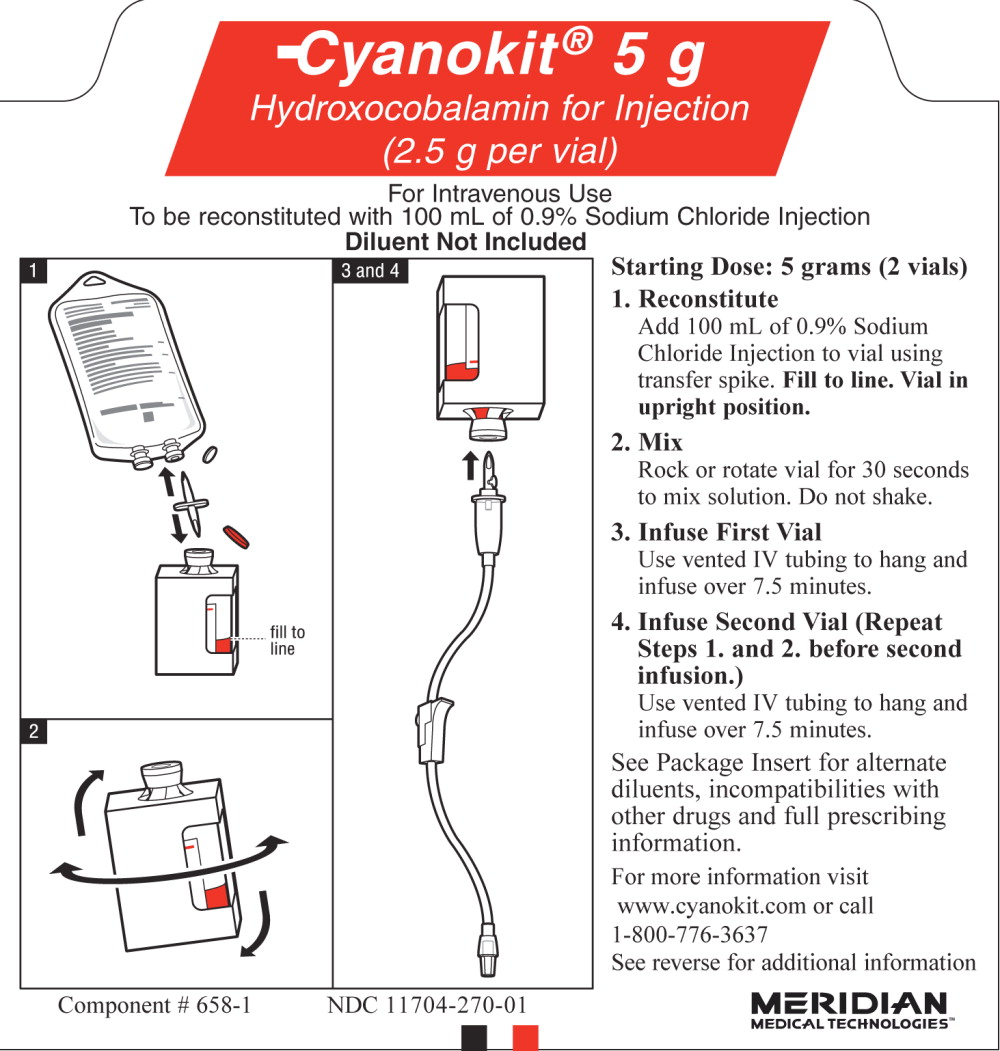
PRINCIPAL DISPLAY PANEL
PACKAGE LABEL – PRINCIPAL DISPLAY PANEL – 5 g PATIENT INSTRUCTION CARD SIDE B