NDC Code(s) : 11980-801-03
Packager : Allergan, Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| OCUFENflurbiprofen sodium SOLUTION/ DROPS | ||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
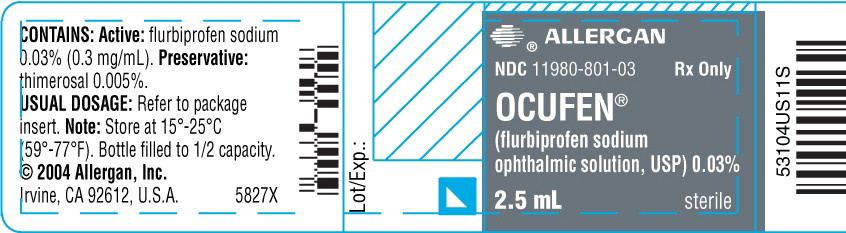
PRINCIPAL DISPLAY PANEL
ALLERGAN
NDC 11980-801-03
Rx Only
OCUFEN®
(flurbiprofen
sodium ophthalmic
solution, USP)
0.03%
sterile
2.5 mL
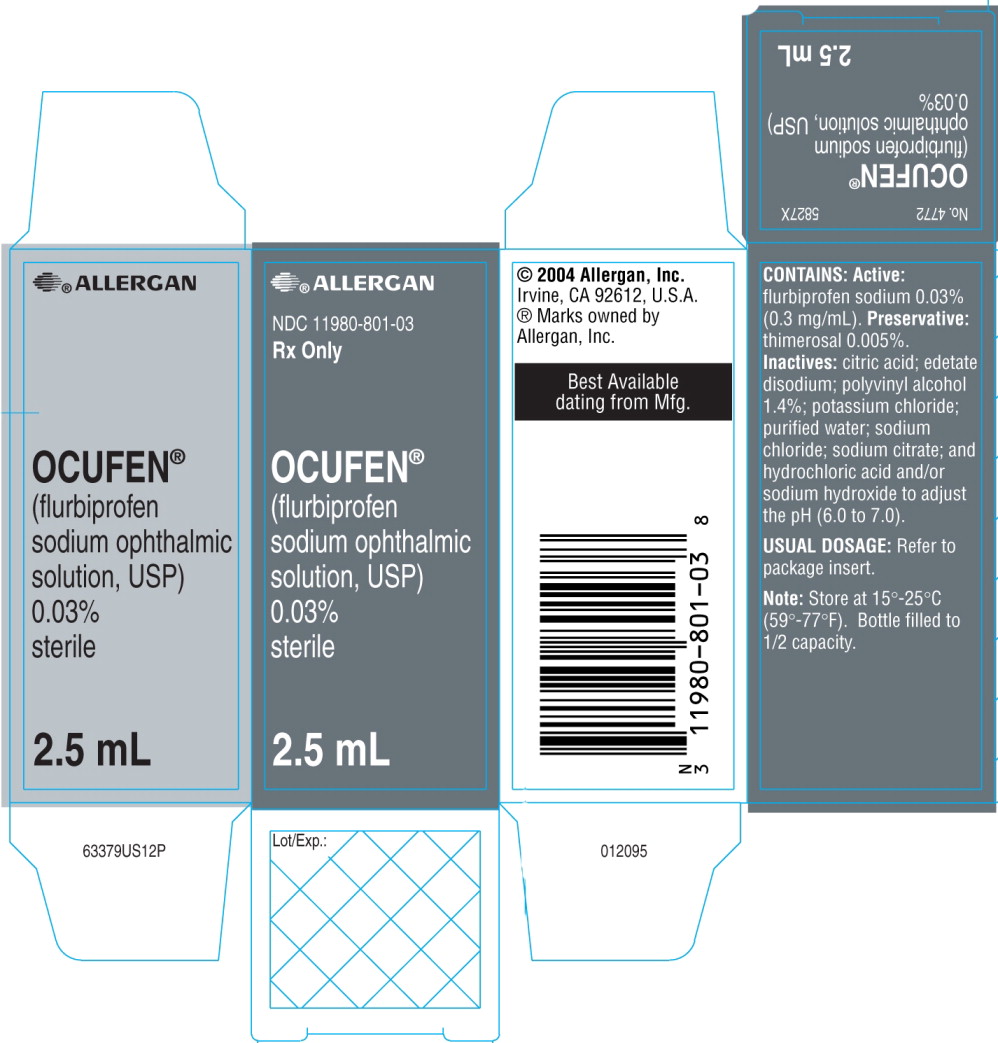
PRINCIPAL DISPLAY PANEL
ALLERGAN
NDC 11980-801-03 Rx Only
OCUFEN®
(flurbiprofen sodium
ophthalmic solution, USP) 0.03%
2.5 mL sterile