NDC Code(s) : 11994-011-04, 11994-011-16, 11994-011-01
Packager : Lantheus Medical Imaging, Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Definityperflutren INJECTION, SUSPENSION | ||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| LABELER - Lantheus Medical Imaging, Inc.(176786812) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Lantheus Medical Imaging, Inc. | 176786812 | ANALYSIS(11994-011), MANUFACTURE(11994-011), PACK(11994-011), LABEL(11994-011) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Jubilant HollisterStier | 069263643 | MANUFACTURE(11994-011) | |
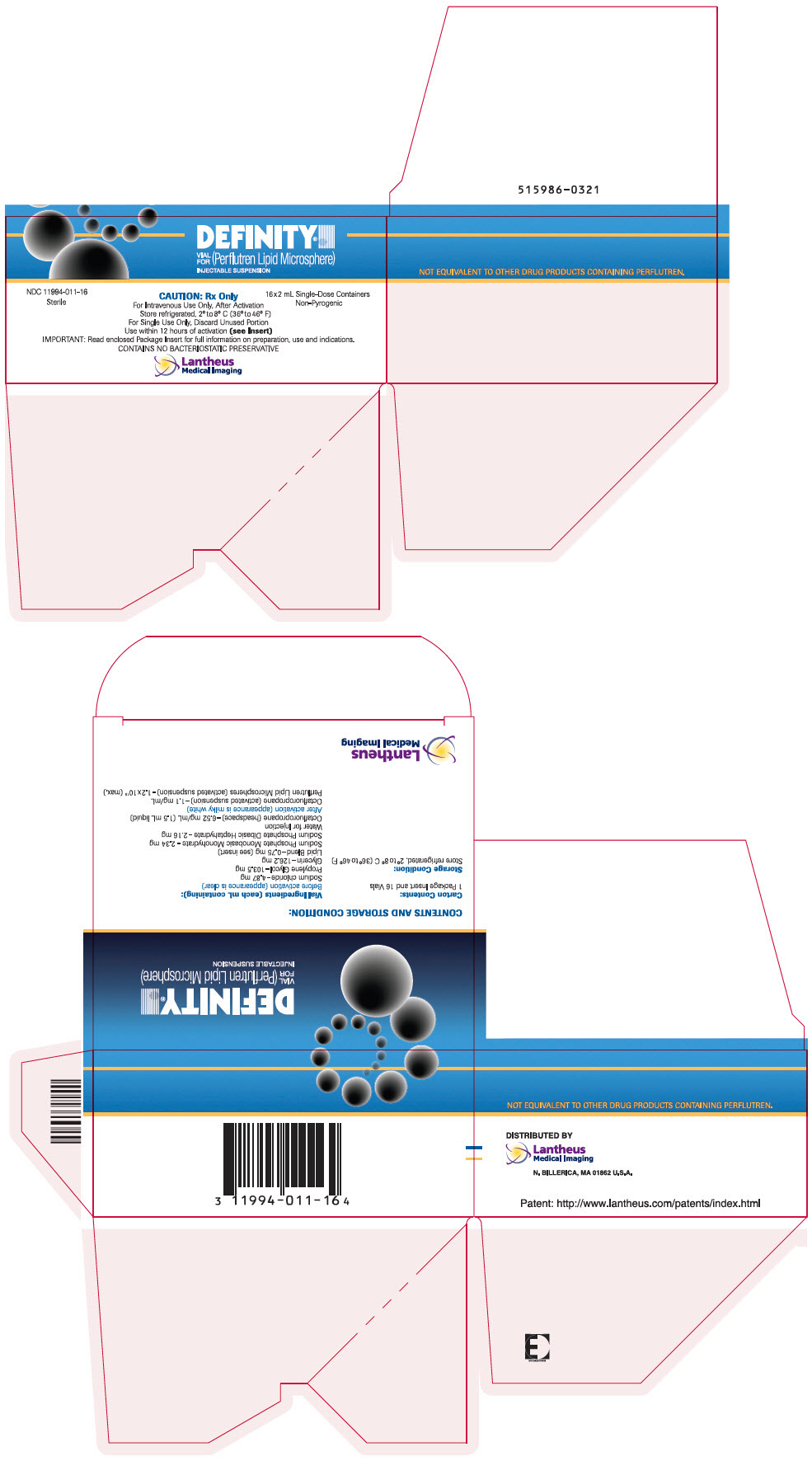
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 2 mL Vial Carton
DEFINITY®
VIAL
FOR
(Perflutren Lipid Microsphere)
INJECTABLE SUSPENSION
NDC 11994-011-16
Sterile
CAUTION: Rx Only
16x2 mL Single-Dose Containers
Non-Pyrogenic
For Intravenous Use Only, After Activation
Store refrigerated, 2° to 8° C (36° to 46° F)
For Single Use Only, Discard Unused Portion
Use within 12 hours of activation (see Insert)
IMPORTANT: Read enclosed Package Insert for full information on preparation, use and indications.
CONTAINS NO BACTERIOSTATIC PRESERVATIVE
Lantheus
Medical Imaging