NDC Code(s) : 12064-014-00, 12064-013-00
Packager : GlaxoSmithKline Manufacturing SpA
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| IMITREXsumatriptan SPRAY | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| IMITREXsumatriptan SPRAY | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
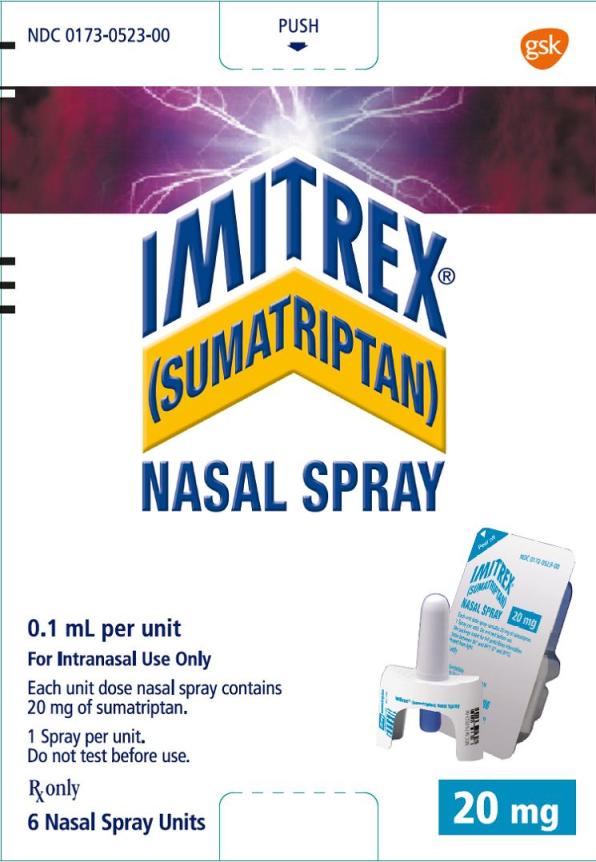
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
NDC 0173-0523-00
IMITREX®
(SUMATRIPTAN)
NASAL SPRAY
20 mg
0.1 mL per unit
For Intranasal Use Only
Each unit dose nasal spray contains 20 mg of sumatriptan.
1 Spray per unit.
Do not test before use.
Rx only
6 Nasal Spray Units
©2015, the GSK group of companies
- 10000000135321 Rev. 6/15
PRINCIPAL DISPLAY PANEL
NDC 0173-0524-00
IMITREX®
(SUMATRIPTAN)
NASAL SPRAY
5 mg
0.1 mL per unit
For Intranasal Use Only
Each unit dose nasal spray contains 5 mg of sumatriptan.
1 Spray per unit.
Do not test before use.
Rx only
6 Nasal Spray Units
©2015, the GSK group of companies
- 10000000135323 Rev. 6/15










