NDC Code(s) : 12064-018-00, 12064-018-01
Packager : GlaxoSmithKline Manufacturing SpA
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Nucalamepolizumab INJECTION, POWDER, FOR SOLUTION | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
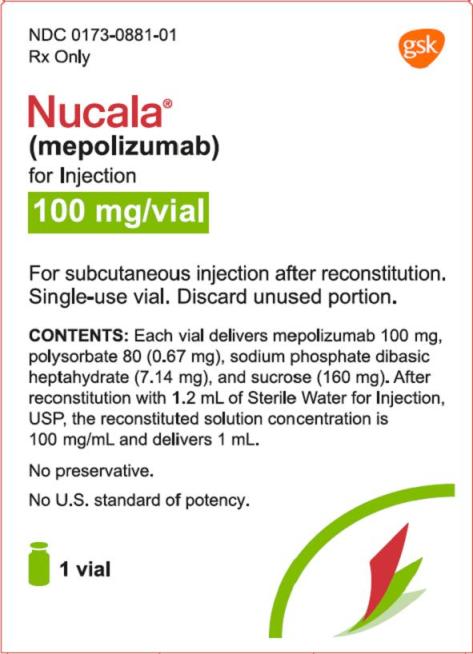
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
NDC 0173-0881-01
Nucala®
(mepolizumab)
for Injection
100 mg/vial
Rx Only
For subcutaneous injection after reconstitution.
Single-use vial. Discard unused portion.
Contents: Each vial delivers mepolizumab 100 mg, polysorbate 80 (0.67 mg), sodium phosphate dibasic heptahydrate (7.14 mg), and sucrose (160 mg). After reconstitution with 1.2 mL of Sterile Water for Injection, USP, the reconstituted solution concentration is 100 mg/mL and delivers 1 mL.
No preservative.
No U.S. standard of potency.
1 vial
Do not accept if plastic overseal is missing or not securely fitted.
©2016 the GSK group of companies
- 10000000141958 Rev. 6/16