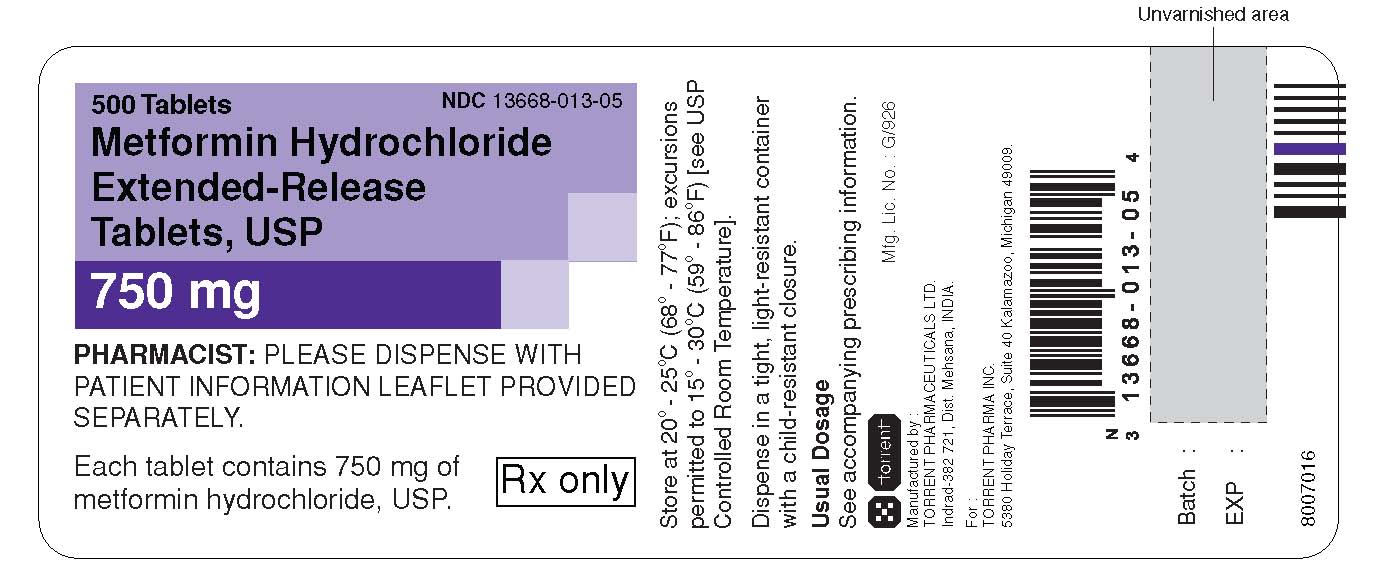
NDC Code(s) : 13668-012-90, 13668-012-60, 13668-012-30, 13668-012-05, 13668-012-12, 13668-013-90, 13668-013-12, 13668-013-05, 13668-013-30, 13668-013-60
Packager : Torrent Pharmaceuticals Limited
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Metformin HydrochlorideMetformin Hydrochloride TABLET, EXTENDED RELEASE | |||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
INGREDIENTS AND APPEARANCE
| Metformin Hydrochloridemetformin hydrochloride TABLET, EXTENDED RELEASE | |||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
PRINCIPAL DISPLAY PANEL
500 mg : label

PRINCIPAL DISPLAY PANEL
750 mg : label