NDC Code(s) : 15455-1032-4
Packager : Alvix Laboratories,LLC
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Lidocaine HydrochlorideLidocaine Hydrochloride LOTION | ||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
PRINCIPAL DISPLAY PANEL
4 FL. OZ (118 mL) bottle
NDC 15455-1032-4
TOPICAL ANESTHETIC
LIDOCAINE
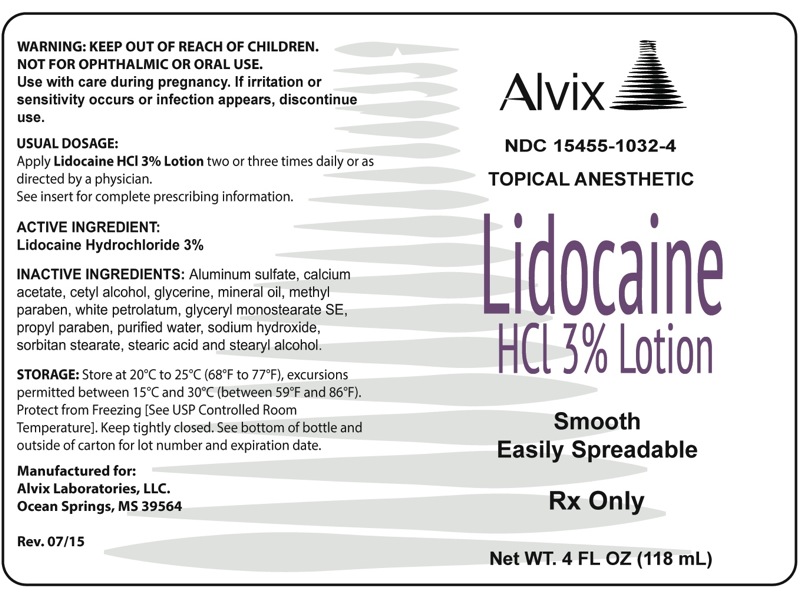
HCl 3% LOTION
Rx only
Net WT. 4 fl. oz. (118 mL)
4 FL. OZ (118 mL) carton
NDC 15455-1032-4

TOPICAL ANESTHETIC
LIDOCAINE
HCl 3% LOTION
Rx only
Net WT. 4 fl. oz. (118 mL)









