NDC Code(s) : 16781-201-06, 16781-201-96
Packager : Onset Dermatologics LLC
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| BenzEFoam UltraBENZOYL PEROXIDE AEROSOL, FOAM | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
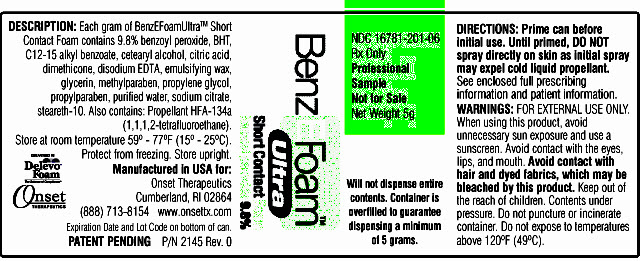
PRINCIPAL DISPLAY PANEL
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - BenzEFoamUltraTM 5 Grams Carton Label
NDC 16781-201-06
Rx Only
BenzEFoamTM Ultra
Short Contact Foam
benzoyl peroxide 9.8%
For topical treatment of mild to moderate acne vulgaris
Onset THERAPEUTICS
Professional Samples
Enclosed: Six 5g Samples
Available in 100g Cans

PRINCIPAL DISPLAY PANEL
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - BenzEFoamUltraTM 5 Grams Can Label
NDC 16781-201-06
Rx Only
Professional Sample
Not for Sale
Net Weight 5g
BenzEFoamTM Ultra
Short contact Foam
benzoyl peroxide 9.8%
Will not dispense entire contents. Container is overfilled to guarantee dispensing a minimum of 5 grams.