NDC Code(s) : 17478-100-02, 17478-100-10, 17478-100-12, 17478-097-02, 17478-097-10, 17478-097-12
Packager : Akorn
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Cyclopentolate HydrochlorideCyclopentolate Hydrochloride SOLUTION/ DROPS | ||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Cyclopentolate HydrochlorideCyclopentolate Hydrochloride SOLUTION/ DROPS | ||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| LABELER - Akorn(117693100) |
PRINCIPAL DISPLAY PANEL
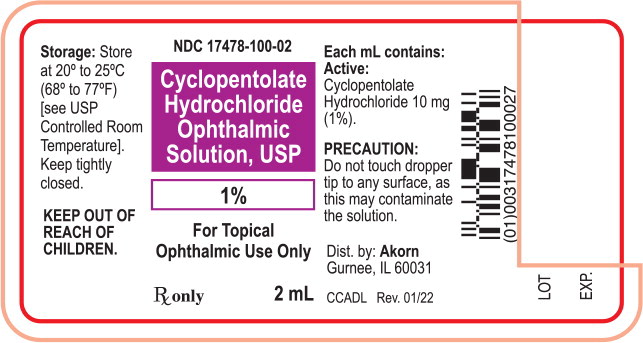
Principal Display Panel Text for Container Label:
NDC 17478-100-02
Cyclopentolate
Hydrochloride
Ophthalmic
Solution, USP
1%
For Topical
Ophthalmic Use Only
Rx only 2 mL
PRINCIPAL DISPLAY PANEL
Principal Display Panel Text for Carton Label:
NDC 17478-100-02
Cyclopentolate
Hydrochloride
Ophthalmic
Solution, USP
1%
For Topical
Ophthalmic Use Only
2 mL
Rx only Akorn logo

PRINCIPAL DISPLAY PANEL
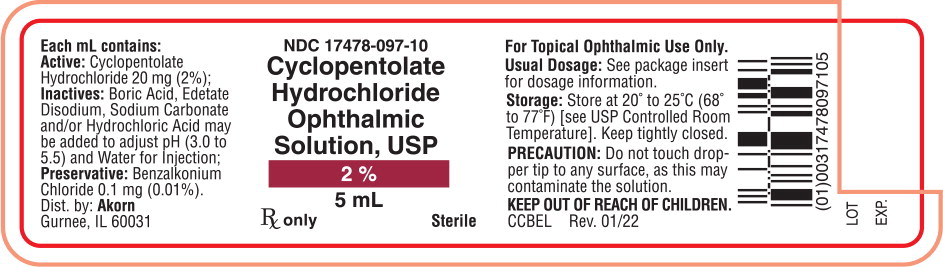
Principal Display Panel Text for Container Label:
NDC 17478-097-10
Cyclopentolate
Hydrochloride
Ophthalmic
Solution, USP
2%
5 mL
Rx only Sterile
PRINCIPAL DISPLAY PANEL
Principal Display Panel Text for Carton Label:
NDC 17478-097-10
Cyclopentolate
Hydrochloride
Ophthalmic
Solution, USP
2%
5 mL
Sterile
Rx only Akorn logo










