NDC Code(s) : 17478-825-05
Packager : Akorn, Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Dehydrated AlcoholAlcohol INJECTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
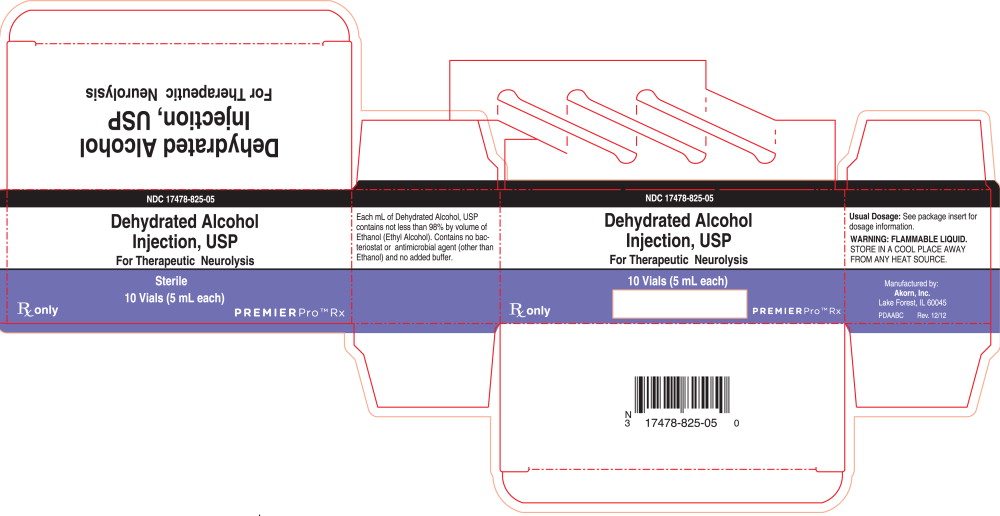
PRINCIPAL DISPLAY PANEL
Principal Display Panel Text for Container Label:
NDC 17478-825-05
Dehydrated Alcohol
Injection, USP
For Therapeutic Neurolysis
Sterile
Rx only 5 mL
Premier Logo

PRINCIPAL DISPLAY PANEL
Principal Display Panel Text for Carton Label:
NDC 17478-825-05
Dehydrated Alcohol
Injection, USP
For Therapeutic Neurolysis
Sterile
10 Vials (5 mL each)
Rx only Premier Logo