NDC Code(s) : 17500-2101-4
Packager : Kingkey Daily Chemical Co LTD
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Dermasil Dimethicone LOTION | ||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
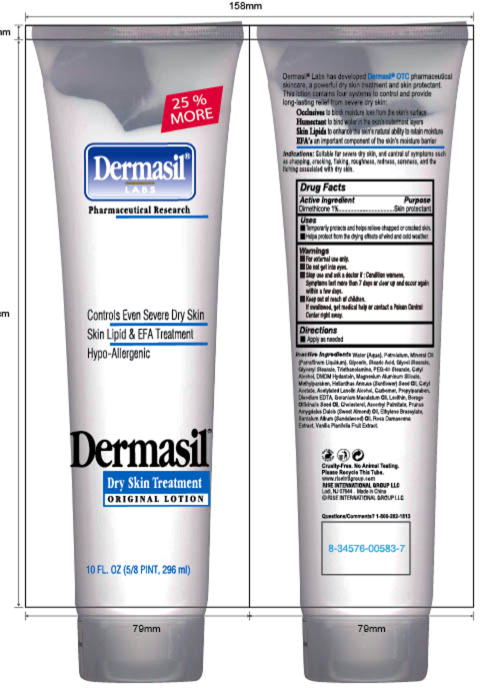
PRINCIPAL DISPLAY PANEL
Principal Display Panel - 10 FL.0Z (5/8 PINT. 296 ml)
Tube Label Dermasil 10oz
25% MORE
Dermasil
®
LABS
Pharmaceutical Research
Controls Even Severe Dry Skin
Skin Lipid & EFA Treatment
Hypo-Allergenic
Dermasil
®
Dry Skin Treatment
ORIGINAL LOTION
10 FL.0Z (5/8 PINT. 296 ml)